Both digestion and absorption happen in which of the following parts of the digestive system?
Gallbladder
Esophagus
Stomach
Small intestine
Correct Answer : D
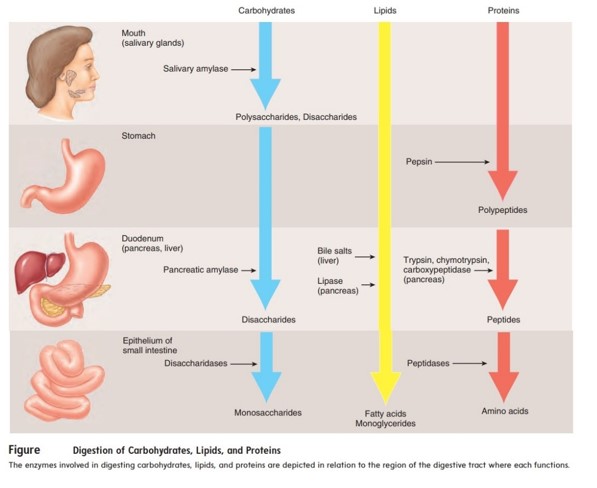
Both digestion and absorption happen in the small intestine ¹. Digestion is the process by which food is broken down into small molecules that the body can absorb and use for energy, growth, and repair ¹. The final products of digestion are absorbed from the digestive tract, primarily in the small intestine ¹.
The other options are not correct because they do not accurately describe where both digestion and absorption happen in the digestive system. The gallbladder stores bile produced by the liver, but does not play a direct role in digestion or absorption. The esophagus transports food from the mouth to the stomach, but does not play a direct role in digestion or absorption. The stomach plays a role in digestion by churning food and mixing it with gastric juices, but most absorption occurs in the small intestine.
TEAS 7 Exam Quiz Bank
HESI A2 Exam Quiz Bank
Find More Questions 📚
Teas 7 Questions: We got the latest updated TEAS 7 questions
100% Money Refund: 100% money back guarantee if you take our full
assessment pass with 80% and fail the actual exam.
Live Tutoring: Fully customized live tutoring lessons.
Guaranteed A Grade: All students who use our services pass with 90%
guarantee.
Related Questions
Correct Answer is B
Explanation
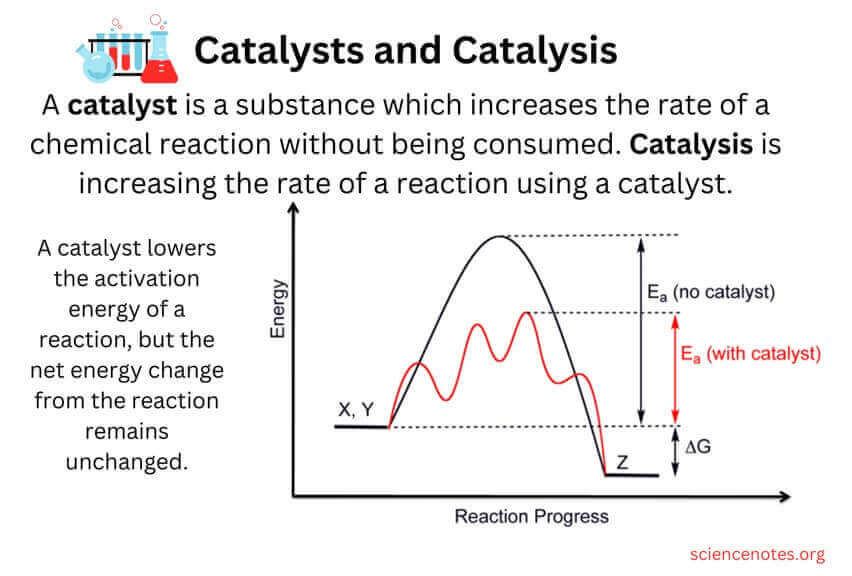
The result of using a catalyst in a chemical reaction is that the reaction is completed in a shorter amount of time ¹. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed ¹. This process is called catalysis ¹. A catalyst provides an alternative pathway for the reaction, one that has a lower activation energy than the uncatalyzed pathway .
The other options are not correct because they do not accurately describe the result of using a catalyst in a chemical reaction. A more desirable product is not necessarily formed, a greater amount of heat energy is not necessarily released by the reaction, and the yield of product is not necessarily increased as a result of using a catalyst.
Correct Answer is C
Explanation
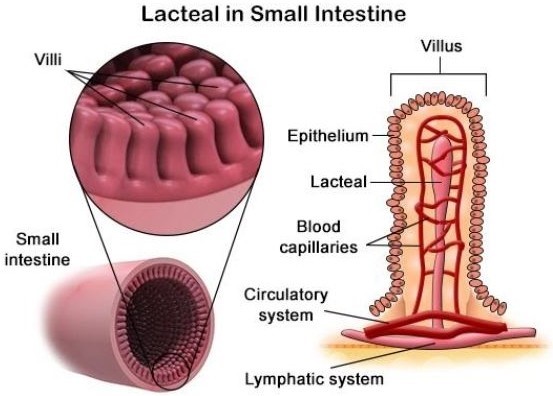
The correct answer is c. Lacteal vessels. Lipids absorbed in the small intestine will first enter lacteal vessels, which are small lymphatic vessels located in the villi of the small intestine. These vessels transport the absorbed lipids to the lymphatic system, where they eventually enter the bloodstream.
a. Veins and b. Arteries are blood vessels that transport blood throughout the body. Lipids absorbed in the small intestine do not directly enter these vessels.
d. Interstitial spaces are spaces between cells and tissues that contain interstitial fluid. Lipids absorbed in the small intestine do not directly enter these spaces.
Correct Answer is D
Explanation
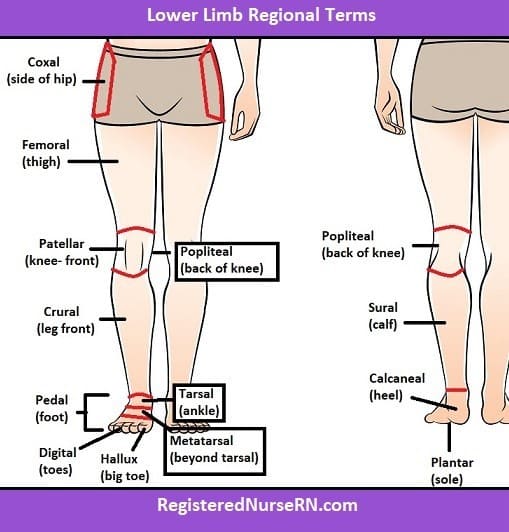
The tibia and fibula are located in the crural region of the body, which is the lower leg between the knee and ankle. The coxal region refers to the hip area, the antecubital region is the front of the elbow, and the tarsal region is the ankle and foot.
Correct Answer is D
Explanation
The apocrine gland is a component of the integumentary system that secretes pheromones. Pheromones are chemical signals that are released by an individual and can affect the behavior or physiology of other individuals of the same species.
The other options are not components of the integumentary system that secrete pheromones. The fossa ovalis is a depression in the interatrial septum of the heart, the seminiferous tubule is a structure in the testes where sperm are produced, and the dermal papilla is a structure at the base of a hair follicle that provides nutrients to the hair.

Correct Answer is B
Explanation
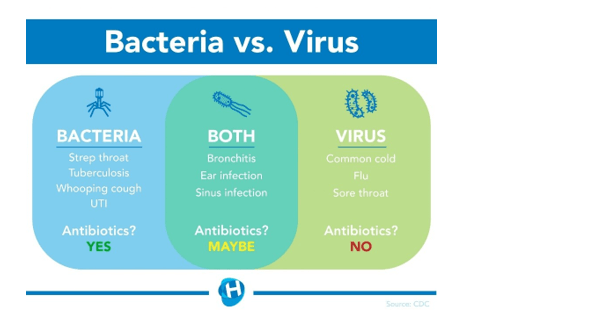
The doctor refuses to prescribe antibiotics to the patient because the illness is caused by a virus. The common cold is a viral infection of the upper respiratory tract. Antibiotics are not effective against viral infections and are only used to treat bacterial infections.
The other options are not correct because they do not accurately describe the cause of the common cold and sore throat. Fungus, protist, and bacteria are not the pathogens responsible for causing the common cold.
Correct Answer is A
Explanation
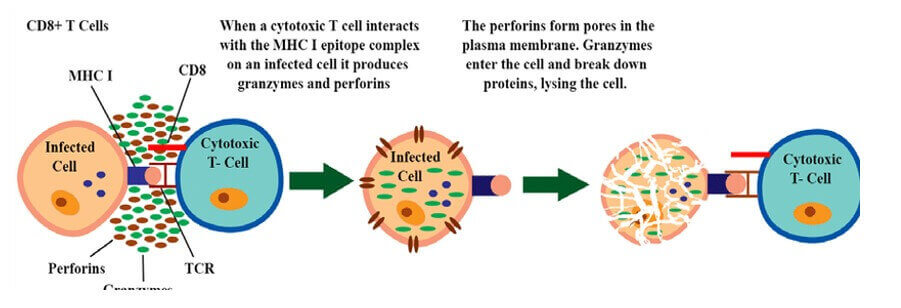
Perforins are immune system molecules that create holes in the cell membranes of their target cells in order to destroy the cell. Perforins are proteins that are released by cytotoxic T cells and natural killer cells
They form pores in the target cell membrane, allowing water and ions to enter the cell and causing it to swell and burst.
The other options are not correct because they do not accurately describe the immune system molecules that create holes in the cell membranes of their target cells. Interferons, cytokines, and lymphotoxins do not create holes in cell membranes.
Correct Answer is C
Explanation
The correct answer is c. Data are biased by the methodology. The researcher's data should be rejected because they are biased by the methodology used to gather them. By only using a small net, the researcher excluded all large birds from the study. This means that the data do not accurately represent the average wing strength of all birds found in the American Northwest.
A. The data contradicting the control group is not a reason to reject the data in this case.
B. The data being different than expected is not a reason to reject the data in this case.
D. The data not being able to be displayed graphically is not a reason to reject the data in this case.
Correct Answer is A
Explanation
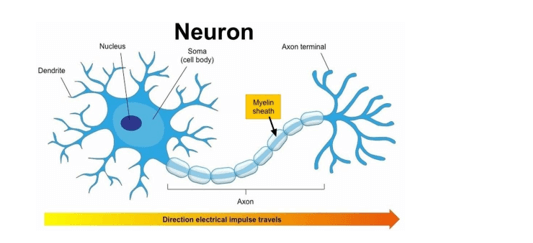
The myelin sheath is a protective membrane that wraps around parts of certain nerve cells.
Its fatty-protein coating provides protective insulation for your nerve cell like the plastic insulation covering that encases the wires of an electrical cord ².
This allows the electrical impulses to travel quickly and efficiently between one nerve cell and the next. The other options are incorrect because they do not describe the functions of the myelin sheath.
Regeneration, sensory perception, and nutrition are not functions performed by the myelin sheath for a nerve cell.
Correct Answer is A
Explanation
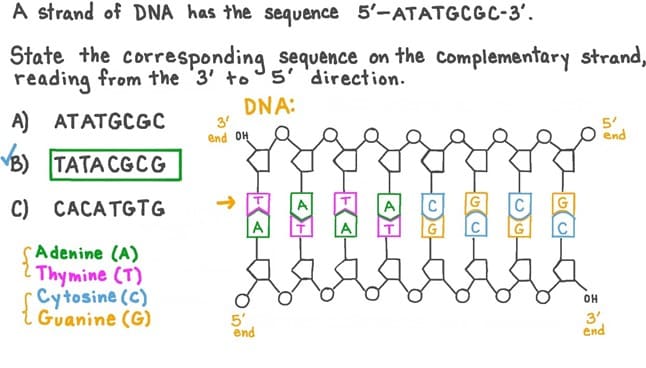
The sequence of bases on the complementary strand of DNA would read5’AGCTAGCGT 3’ (Choice A). In DNA, the nitrogenous bases adenine (A) and thymine (T) pair together, and cytosine (C) and guanine (G) pair together. The complementary strand is also antiparallel to the original strand, meaning that it runs in the opposite direction with the 5' end matching up with the 3' end of the original strand.
The other options do not accurately represent the complementary sequence of bases or the antiparallel orientation of the strands.
BONUS:
Correct Answer is A
Explanation
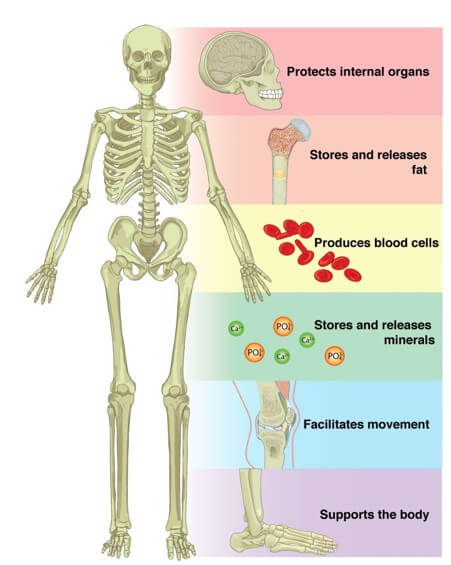
The correct answer is a. Storage of minerals. Bones serve as a storage site for minerals such as calcium and phosphorus. These minerals are essential for various bodily functions and can be released from the bones into the bloodstream when needed.
b. Detoxification of alcohol is not a function of bone. This process occurs primarily in the liver.
c. Secretion of hormones is not a function of bone. Hormones are produced and secreted by glands such as the pituitary gland, thyroid gland, and adrenal glands.
d. Production of otoliths is not a function of bone. Otoliths are small calcium carbonate structures found in the inner ear of fish and other vertebrates that help with balance and hearing.
This question was extracted from the actual TEAS Exam. Ace your TEAS exam with the actual TEAS 7 questions, Start your journey with us today
Visit Naxlex, the Most Trusted TEAS TEST Platform With Guaranteed Pass of 90%.
Money back guarantee if you use our service and fail the actual exam. Option of personalised live tutor on your area of weakness.
