A spoonful of sugar is added to a hot cup of tea. All the sugar dissolves. How can the resulting solution be described?
Saturated and homogeneous
Saturated and heterogeneous
Unsaturated and homogeneous
Unsaturated and heterogeneous
Correct Answer : C
Because more solute could be added and dissolve, the solution has not yet reached its limit and is considered unsaturated. Because all the solute dissolves, the particles in the mixture are evenly distributed as a homogenous mixture.
- A mixture is when elements and compounds are physically, but not chemically, combined.
- A homogeneous mixture is when substances mix evenly and it is impossible to see individual components. A heterogeneous mixture is when the substances mix unevenly and it is possible to see individual components.
- A solution is a type of homogeneous mixture that is formed when a solute dissolves in a solvent.
- The concentration of a solution is the amount of a substance in a given amount of solution. An unsaturated solution has the ability to dissolve more solute and a saturated solution has already reached the limit of solute it can dissolve.
TEAS 7 Exam Quiz Bank
HESI A2 Exam Quiz Bank
Find More Questions 📚
Teas 7 Questions: We got the latest updated TEAS 7 questions
100% Money Refund: 100% money back guarantee if you take our full
assessment pass with 80% and fail the actual exam.
Live Tutoring: Fully customized live tutoring lessons.
Guaranteed A Grade: All students who use our services pass with 90%
guarantee.
Related Questions
Correct Answer is A
Explanation
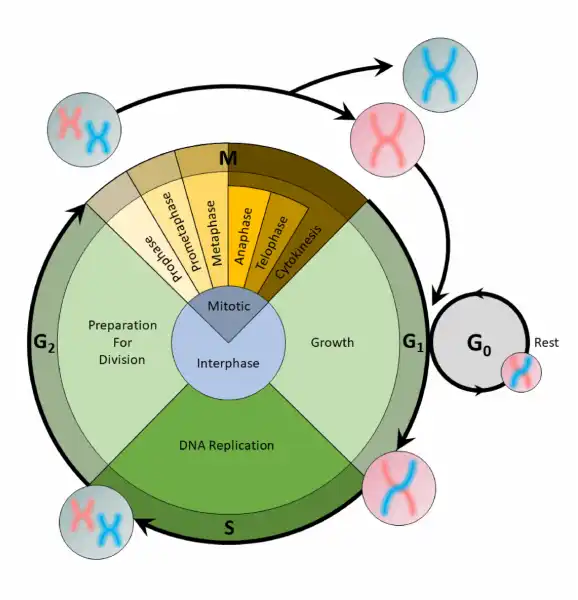
Before mitosis or meiosis occurs, interphase must happen. This is when the cell cycle takes place. The cell cycle is an organized process divided into two phases:interphaseand theM (mitotic) phase. During interphase, the cell grows and copies its DNA. After the cell reaches the M phase, division of the two new cells can occur. The G1, S, and G2phases make up interphase.
Correct Answer is D
Explanation
Because this is a positive correlation, if the nutrient concentration increases or decreases, plant height will either increase or decrease accordingly.
While analyzing data, scientists tend to observe cause-and-effect relationships. These relationships can be quantified using correlations.Correlationsmeasure the amount of linear association between two variables. There are three types of correlations:
Positive correlation:
As one variable increases, the other variable also increases. This is also known as a direct correlation.
Negative correlation:
As one variable increases, the other decreases. The opposite is true if one variable decreases. A negative correlation is also known as an inverse correlation or an indirect correlation.
No correlation:
There is no connection or relationship between two variables.
Correct Answer is D
Explanation
A neutralization reaction is a type of acid-base reaction where an acid and base react to form a salt and water.
In an aqueous solution, a base increases the hydroxide concentration (OH–), while an acid increases the hydrogen ion (H+) concentration. Sometimes,neutralization reactionsalso occur. This type of reaction happens when an acid and a base react with each other to form water and salt. Salt is typically defined as anionic compoundthat includes any cation except H+and any anion except OH–. Consider the following example of a neutralization reaction between hydrobromic acid (HBr) and potassium hydroxide (KOH).
HBr+KOH→KBr+H2O
Not all neutralization reactions proceed in the manner where all reactants are in the aqueous phase. In some chemical reactions, one reactant may be a solid. The neutralization reaction can still proceed to completion.
Correct Answer is D
Explanation
The cardiovascular system is closely associated with hematopoiesis because it includes the heart and blood vessels, which are responsible for circulating blood throughout the body. Hematopoiesis, the process of blood cell formation, primarily occurs in the bone marrow, which is part of the skeletal system. However, the cardiovascular system plays a crucial role in transporting these blood cells to various parts of the body once they are produced in the bone marrow.
So, while the skeletal system provides the site for hematopoiesis, the cardiovascular system is responsible for distributing the blood cells, making it the most closely associated system in this context.
Correct Answer is D
Explanation
In this reaction, two elements are trading places hence double-replacement. In the reactants, zinc and bromide ions are together, and potassium and hydroxide ions are together. In the products, zinc and hydroxide ions are together, and potassium and bromide ions are together.
Correct Answer is A
Explanation
After scientists were able to view cells under the microscope they formulated the cell theory. One part of this theory concluded that all cells are alive. They also represent the basic unit of life.
All living things are made of cells. Cells are the smallest structural units and basic building blocks of living things. Cells contain everything necessary to keep living things alive. Varying in size and shape, cells carry out specialized functions. This theory, or in-depth explanation, about cells consists of three parts:
- All living things are composed of one or more cells.
- Cells are alive and represent the basic unit of life.
- All cells are produced from pre-existing cells.
Correct Answer is B
Explanation
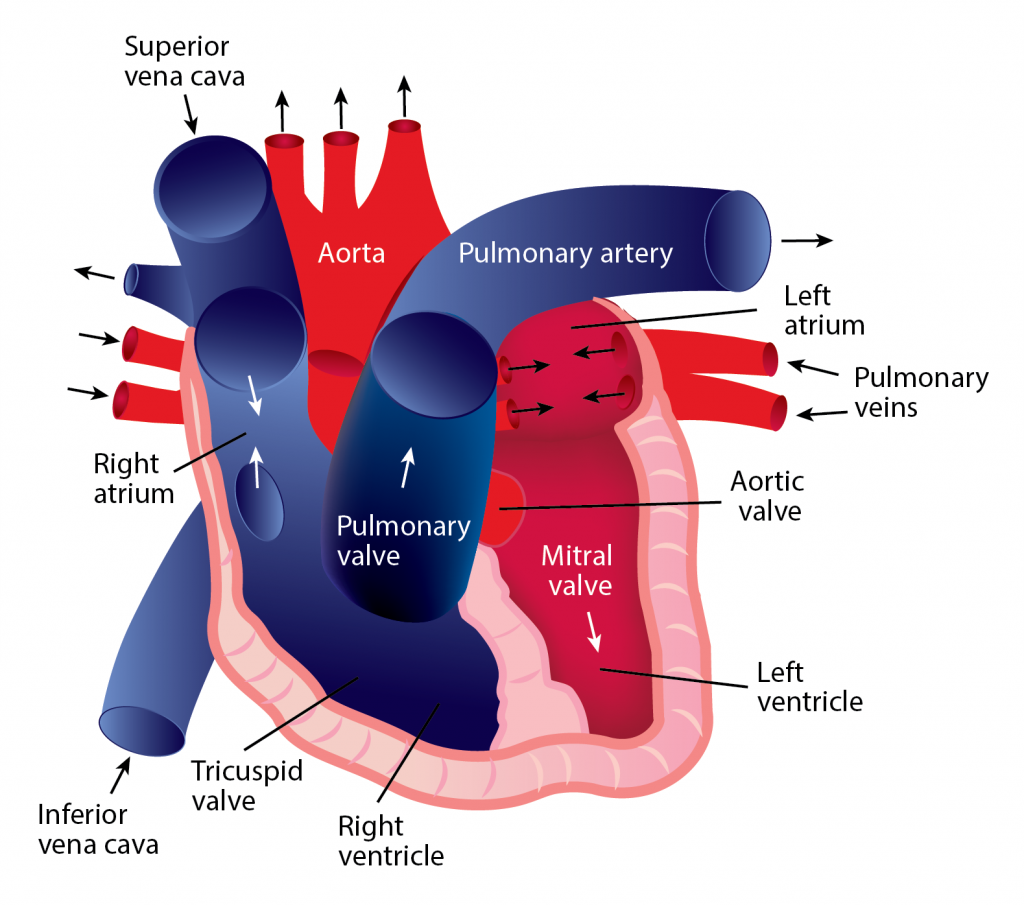
Blood continually flows in one direction, beginning in the heart and proceeding to the arteries, arterioles, and capillaries. When blood reaches the capillaries, exchanges occur between blood and tissues. After this exchange happens, blood is collected into venules, which feed into veins and eventually flow back to the heart’s atrium. The heart must relax between two heartbeats for blood circulation to begin.
Two types of circulatory processes occur in the body:
Systemic circulation
- The pulmonary vein pushes oxygenated blood into the left atrium.
- As the atrium relaxes, oxygenated blood drains into the left ventricle through the mitral valve. 3. The left ventricle pumps oxygenated blood to the aorta.
- Blood travels through the arteries and arterioles before reaching the capillaries that surround the tissues.
Pulmonary circulation
- Two major veins, the Superior Vena Cava and the Inferior Vena Cava, brings deoxygenated blood from the upper and lower half of the body.
- Deoxygenated blood is pooled into the right atrium and then sent into the right ventricle through the tricuspid valve, which prevents blood from flowing backward.
- The right ventricle contracts, causing the blood to be pushed through the pulmonary valve into the pulmonary artery.
- Deoxygenated blood becomes oxygenated in the lungs.
- Oxygenated blood returns from the lungs to the left atrium through the pulmonary veins.
Correct Answer is B
Explanation
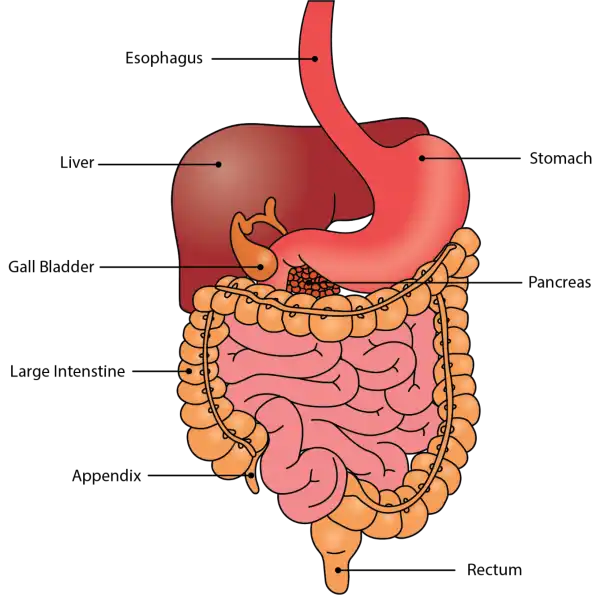
Oral Cavityis the first part of the digestive system. It is bounded by the lips and cheeks and contains the teeth and tongue. Its primary function is to masticate, or chew, and moisten the food.
Pharynx, or throat, connects the mouth to the esophagus.
Esophagusis a muscular tube about 25 centimeters long. Food travels down it to the cardiac sphincter of the stomach.
Pyloric sphincter. The exit of the stomach.
Small intestineis about 6 meters long and consists of three parts: duodenum, jejunum, and ileum.
Large intestine, consists of the cecum, colon, rectum, and anal canal. The cecum is located where the small and large intestine meet. The primary function of the large intestine is to compress the waste and collect any excess water that can be recycled.
Colonis about 1.5 to 1.8 meters long and consists of four parts: the ascending, transverse, descending, and sigmoid colon.
Correct Answer is C
Explanation
One step of the scientific method is to analyze information or data collected from the experiment to conclude whether the hypothesis is supported.
Recall that these make up thescientific method,described below:
- Problem:The question created because of an observation.Example: Does the size of a plastic object affect how fast it naturally degrades in a lake?
- Research:Reliable information available about what is observed.Example: Learn how plastics are made and understand the properties of a lake.
- Hypothesis:A predicted solution to the question or problem.Example: If the plastic material is small, then it will degrade faster than a large particle.
- Experiment:A series of tests used to evaluate the hypothesis. Experiments consist of anindependent variablethat the researcher modifies and adependent variablethat changes due to the independent variable. They also include acontrol groupused as a standard to make comparisons.
- Example: Collect plastic particles both onshore and offshore of the lake over time. Determine the size of the particles and describe the lake conditions during this time period.
- Observe:Analyze data collected during an experiment to observe patterns.
- Example: Analyze the differences between the numbers of particles collected in terms of size.
- Conclusion:State whether the hypothesis is rejected or accepted and summarize all results.
- Communicate:Report findings so others can replicate and verify the results.
Correct Answer is A
Explanation
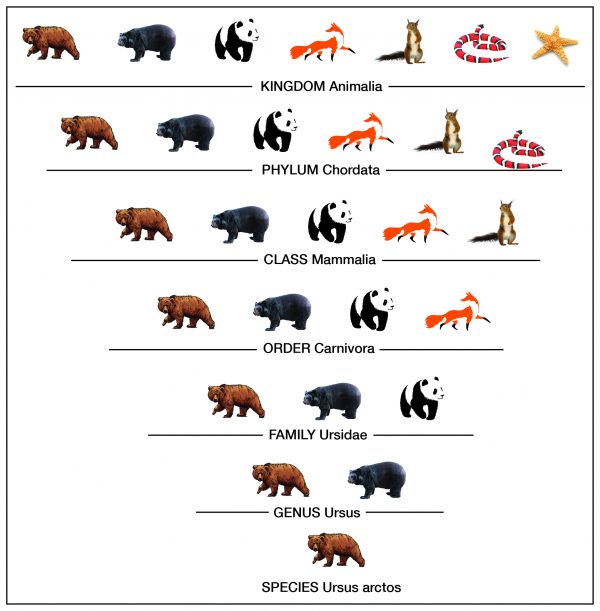
Taxonomy is the process of classifying, describing, and naming organisms. There are seven levels in the Linnaean taxonomic system, starting with the broadest level, kingdom, and ending with the species level. For example, in the image the genus level contains two types of bears, but the species level shows one type. Additionally,organisms in each level are found in the level above it. For example, organisms in the order level are part of the class level. This classification system is based on physical similarities across living things. It does not account for molecular or genetic similarities.
This question was extracted from the actual TEAS Exam. Ace your TEAS exam with the actual TEAS 7 questions, Start your journey with us today
Visit Naxlex, the Most Trusted TEAS TEST Platform With Guaranteed Pass of 90%.
Money back guarantee if you use our service and fail the actual exam. Option of personalised live tutor on your area of weakness.
