In which state of matter are the intermolecular forces between particles in a substance the strongest?
Gas
Liquid
Plasma
Solid
Correct Answer : D
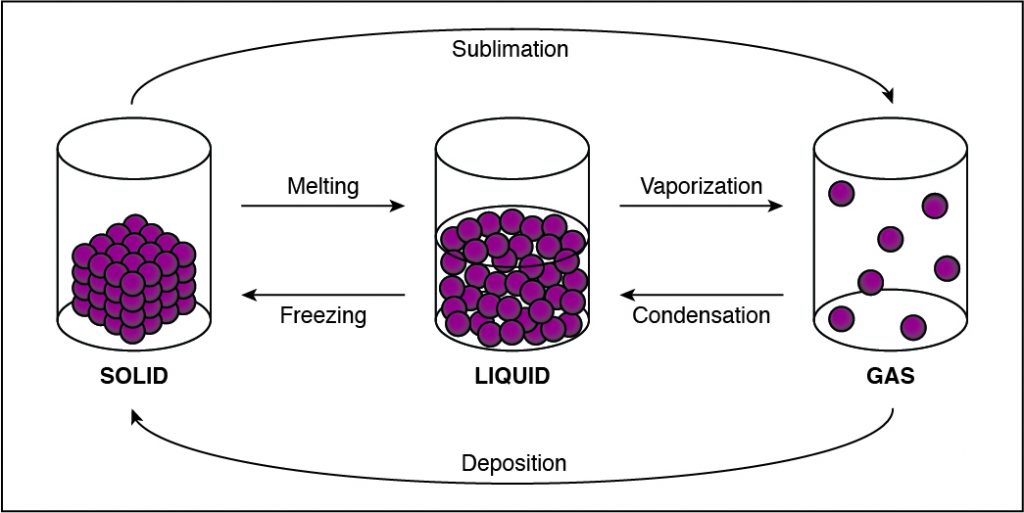
In solids, particles are usually closer together than in other states of matter because of the strong cohesive forces between the particles.
- Solids, liquids, gases, and plasmas differ from one another in the amount of energy that the particles have and the strength of the cohesive forces that hold the particles together.
- Cohesion is the tendency of particles of the same kind to stick to each other.
- A solid has the lowest amount of energy because its particles are packed close together. Liquids have more energy than a solid, and gases have more energy than solids or liquids because the cohesive forces are very weak.
TEAS 7 Exam Quiz Bank
HESI A2 Exam Quiz Bank
Find More Questions 📚
Teas 7 Questions: We got the latest updated TEAS 7 questions
100% Money Refund: 100% money back guarantee if you take our full
assessment pass with 80% and fail the actual exam.
Live Tutoring: Fully customized live tutoring lessons.
Guaranteed A Grade: All students who use our services pass with 90%
guarantee.
Related Questions
Correct Answer is A
Explanation
After scientists were able to view cells under the microscope they formulated the cell theory. One part of this theory concluded that all cells are alive. They also represent the basic unit of life.
All living things are made of cells. Cells are the smallest structural units and basic building blocks of living things. Cells contain everything necessary to keep living things alive. Varying in size and shape, cells carry out specialized functions. This theory, or in-depth explanation, about cells consists of three parts:
- All living things are composed of one or more cells.
- Cells are alive and represent the basic unit of life.
- All cells are produced from pre-existing cells.
Correct Answer is A
Explanation
Robert Hooke discovered the first cells in the mid-eighteenth century. The cell theory is a theory because it is supported by a significant number of experimental findings. The cell theory took many years to be developed because microscopes were not powerful enough to make such observations.
This theory, or in-depth explanation, about cells consists of three parts:
- All living things are composed of one or more cells.
- Cells are alive and represent the basic unit of life.
- All cells are produced from pre-existing cells.
Correct Answer is B
Explanation
A cell copies its DNA during the S phase, and nucleotides are the building blocks of DNA. Thus, the step preceding the S phase, theG1phase, is the phase of the cell cycle when the cell would contain the most nucleotides.
For a cell to divide into more cells, it must grow, copy its DNA, and produce new daughter cells. Thecell cycleregulates cellular division. This process can either prevent a cell from dividing or trigger it to start dividing.
The cell cycle is an organized process divided into two phases:interphaseand theM (mitotic) phase. During interphase, the cell grows and copies its DNA. After the cell reaches the M phase, division of the two new cells can occur. The G1, S, and G2phases make up interphase.
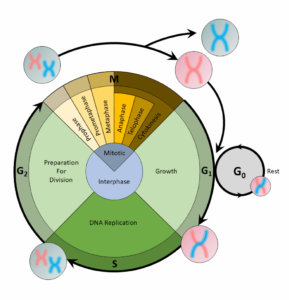
- G1:The first gap phase, during which the cell prepares to copy its DNA
- S:The synthesis phase, during which DNA is copied
- G2:The second gap phase, during which the cell prepares for cell division
It may appear that little is happening in the cell during the gap phases. Most of the activity occurs at the level of enzymes and macromolecules. The cell produces things like nucleotidesfor synthesizing new DNA strands, enzymes for copying the DNA, and tubulin proteins for building the mitotic spindle. During the S phase, the DNA in the cell doubles, but few other signs are obvious under the microscope. All the dramatic events that can be seen under a microscope occur during the M phase: the chromosomes move, and the cell splits into two new cells with identical nuclei.
Correct Answer is D
Explanation
The cardiovascular system is closely associated with hematopoiesis because it includes the heart and blood vessels, which are responsible for circulating blood throughout the body. Hematopoiesis, the process of blood cell formation, primarily occurs in the bone marrow, which is part of the skeletal system. However, the cardiovascular system plays a crucial role in transporting these blood cells to various parts of the body once they are produced in the bone marrow.
So, while the skeletal system provides the site for hematopoiesis, the cardiovascular system is responsible for distributing the blood cells, making it the most closely associated system in this context.
Correct Answer is D
Explanation
A neutralization reaction is a type of acid-base reaction where an acid and base react to form a salt and water.
In an aqueous solution, a base increases the hydroxide concentration (OH–), while an acid increases the hydrogen ion (H+) concentration. Sometimes,neutralization reactionsalso occur. This type of reaction happens when an acid and a base react with each other to form water and salt. Salt is typically defined as anionic compoundthat includes any cation except H+and any anion except OH–. Consider the following example of a neutralization reaction between hydrobromic acid (HBr) and potassium hydroxide (KOH).
HBr+KOH→KBr+H2O
Not all neutralization reactions proceed in the manner where all reactants are in the aqueous phase. In some chemical reactions, one reactant may be a solid. The neutralization reaction can still proceed to completion.
Correct Answer is C
Explanation
One step of the scientific method is to analyze information or data collected from the experiment to conclude whether the hypothesis is supported.
Recall that these make up thescientific method,described below:
- Problem:The question created because of an observation.Example: Does the size of a plastic object affect how fast it naturally degrades in a lake?
- Research:Reliable information available about what is observed.Example: Learn how plastics are made and understand the properties of a lake.
- Hypothesis:A predicted solution to the question or problem.Example: If the plastic material is small, then it will degrade faster than a large particle.
- Experiment:A series of tests used to evaluate the hypothesis. Experiments consist of anindependent variablethat the researcher modifies and adependent variablethat changes due to the independent variable. They also include acontrol groupused as a standard to make comparisons.
- Example: Collect plastic particles both onshore and offshore of the lake over time. Determine the size of the particles and describe the lake conditions during this time period.
- Observe:Analyze data collected during an experiment to observe patterns.
- Example: Analyze the differences between the numbers of particles collected in terms of size.
- Conclusion:State whether the hypothesis is rejected or accepted and summarize all results.
- Communicate:Report findings so others can replicate and verify the results.
Correct Answer is D
Explanation
A sensory nerveis a nerve that carries sensory signals from the external environment to the brain to the central nervous system. It is also an afferent nerve, long dendrites of sensory neurons, which sends sensory information towards the central nervous system (CNS). This information is what is sensed, using the five senses from external environment, sight, sound, smell, taste, and touch.
Motor nerveshave onlyefferent fibers, long axons of motor neurons, that carry impulses away from the CNS to the effectors, which are typically tissues and muscles of the body.
Interneuronsarenerve cellsthat act as a bridge between motor and sensory neurons in the CNS. These neurons help form neural circuits, which helps neurons communicate with each other.
Correct Answer is B
Explanation
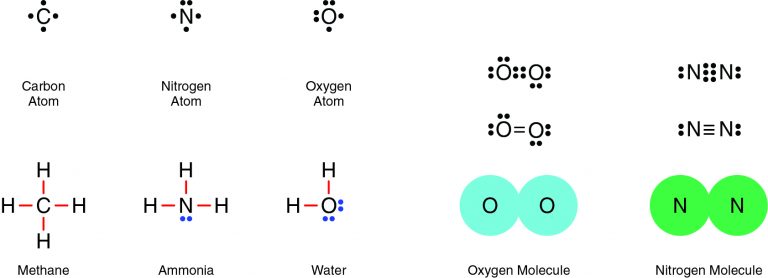
Nitrogen and oxygen are both nonmetals, which means they will share electrons in a covalent bond. For example, two oxygen atoms form a double bond, in which two pairs of electrons (four electrons total) are shared. Similarly, two nitrogen atoms form a molecule with a triple bond, in which three pairs of electrons (six electrons total) are shared.
Correct Answer is B
Explanation
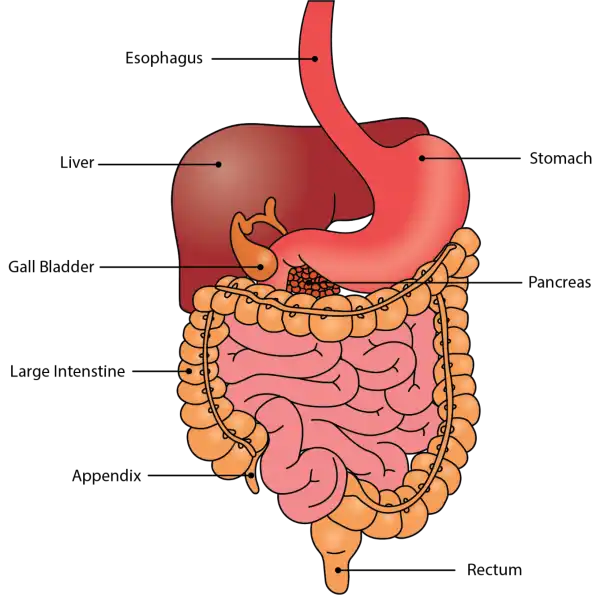
Oral Cavityis the first part of the digestive system. It is bounded by the lips and cheeks and contains the teeth and tongue. Its primary function is to masticate, or chew, and moisten the food.
Pharynx, or throat, connects the mouth to the esophagus.
Esophagusis a muscular tube about 25 centimeters long. Food travels down it to the cardiac sphincter of the stomach.
Pyloric sphincter. The exit of the stomach.
Small intestineis about 6 meters long and consists of three parts: duodenum, jejunum, and ileum.
Large intestine, consists of the cecum, colon, rectum, and anal canal. The cecum is located where the small and large intestine meet. The primary function of the large intestine is to compress the waste and collect any excess water that can be recycled.
Colonis about 1.5 to 1.8 meters long and consists of four parts: the ascending, transverse, descending, and sigmoid colon.
Correct Answer is B
Explanation
The sequence of amino acids in a gene determines the primary structure of a protein. The components necessary fortranslationare located in the cytoplasm. Translation is the making of proteins by mRNA binding to a ribosome with the start codon that initiates the production of amino acids. Apeptide bondforms and connects the amino acids together. The sequence of amino acids determines the protein’s structure, which determines its function.
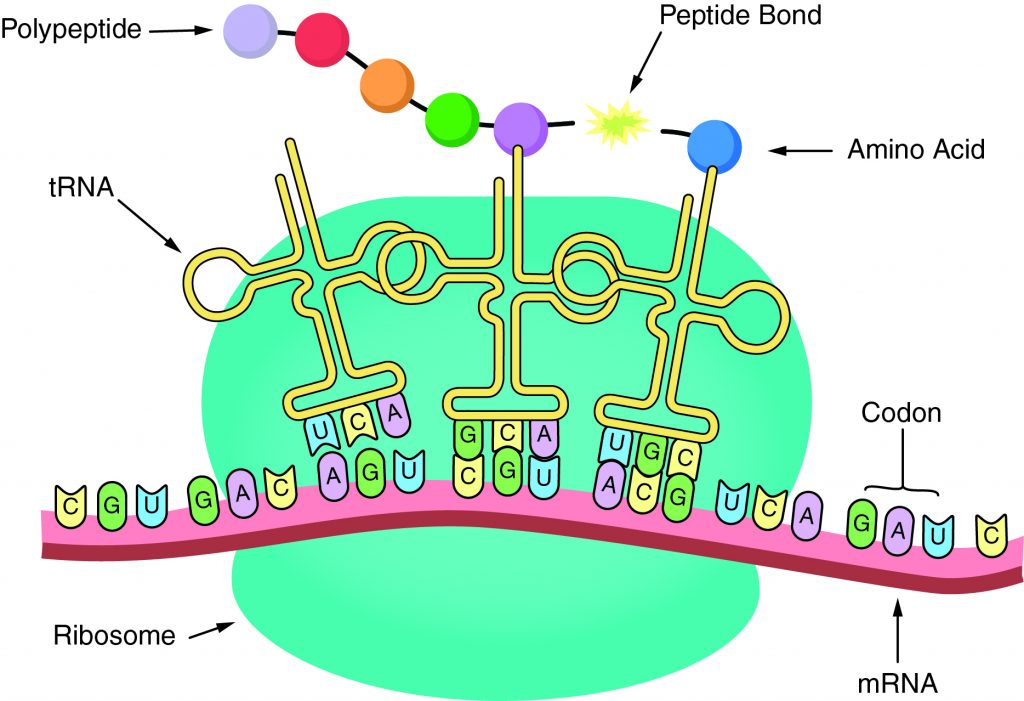
This question was extracted from the actual TEAS Exam. Ace your TEAS exam with the actual TEAS 7 questions, Start your journey with us today
Visit Naxlex, the Most Trusted TEAS TEST Platform With Guaranteed Pass of 90%.
Money back guarantee if you use our service and fail the actual exam. Option of personalised live tutor on your area of weakness.
