What is the difference between isotonic and isometric muscle contractions?
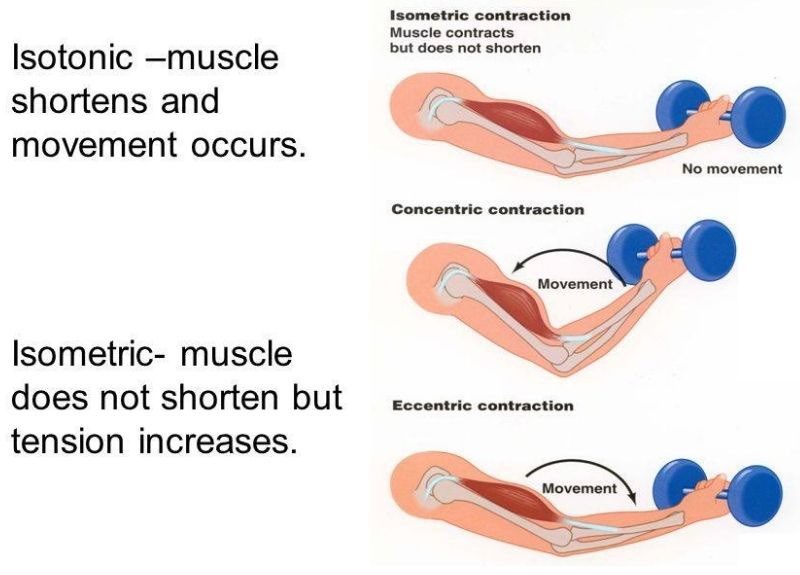
Isotonic contractions produce no movement while isometric contractions produce movement.
Isotonic contractions produce movement while isometric contractions produce no movement.
Isotonic contractions generate tension in the muscle while isometric contractions involve shortening of the muscle fibers.
Isotonic contractions involve contraction of individual muscle fibers while isometric contractions involve the entire muscle.
Correct Answer : B
Isotonic and isometric contractions are two types of muscle contractions that differ in the amount of force produced and the movement of the muscle. In isotonic contractions, the muscle changes length and produces movement, such as lifting a weight. The force generated by the muscle remains constant throughout the movement. Isotonic contractions can be further classified as concentric contractions, in which the muscle shortens as it contracts, and eccentric contractions, in which the muscle lengthens as it contracts.
In contrast, isometric contractions occur when the muscle generates force without changing its length or producing movement. For example, holding a weight in a fixed position without moving it requires an isometric contraction. In an isometric contraction, the force generated by the muscle increases up to a maximum and then remains constant. Isometric contractions can be used to build strength and endurance in the muscle, but they do not produce movement.
 |
TEAS 7 Exam Quiz Bank
HESI A2 Exam Quiz Bank
Find More Questions 📚
Teas 7 Questions: We got the latest updated TEAS 7 questions
100% Money Refund: 100% money back guarantee if you take our full
assessment pass with 80% and fail the actual exam.
Live Tutoring: Fully customized live tutoring lessons.
Guaranteed A Grade: All students who use our services pass with 90%
guarantee.
Related Questions
Correct Answer is C
Explanation
Water molecules primarily enter cells through the process of facilitated diffusion, specifically via aquaporins, which are specialized channel proteins that facilitate the rapid transport of water across the cell membrane. This process does not require energy (ATP) as it relies on the concentration gradient of water, allowing water to move from areas of higher concentration to lower concentration.
Here’s why the other options are not correct in the context of water transport:
- A. Gated channels: While aquaporins can be gated, this term generally refers to channels that open and close in response to specific signals, which is not the primary mechanism for water transport in most cells.
- B. Electrochemical gradients: This term relates to the combined effect of electrical and chemical gradients across a membrane, typically for ions rather than water molecules directly. Water movement can be influenced by osmotic gradients but is not solely dependent on electrochemical gradients.
- D. Proton pumps: These are involved in transporting protons (H⁺ ions) across membranes, primarily for establishing an electrochemical gradient, not for the transport of water.
Thus, water molecules enter cells mainly by facilitated diffusion through aquaporins.
Correct Answer is C
Explanation
Spirometry is a common pulmonary function test that measures pulmonary ventilation, specifically assessing the volume and flow of air that can be inhaled and exhaled from the lungs. It provides important information about lung function and can help diagnose various respiratory conditions.
The other options do not relate to spirometry:
- A. Urinary capacity of the bladder: This is measured by urodynamics or bladder capacity tests, not spirometry.
- B. Volume of blood in the body: This can be estimated using different methods, such as dilution techniques or imaging, but not spirometry.
- D. Number of turns in the small intestine: This relates to the anatomy and function of the digestive system and is not measured by spirometry.
Thus, spirometry specifically evaluates how well the lungs are functioning in terms of air movement.
Correct Answer is C
Explanation
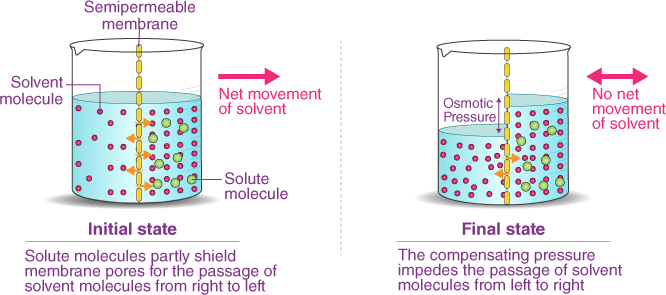
Osmosis is the process by which water molecules move across a selectively permeable membrane from an area of high concentration to an area of low concentration, in order to equalize the concentration of solutes on both sides of the membrane. Selectively permeable membranes allow only certain molecules to pass through, while preventing the passage of others.
In osmosis, the movement of water molecules is driven by the concentration gradient of solutes, which cannot pass through the membrane. If one side of the membrane has a higher concentration of solutes than the other, water molecules will move from the side with the lower concentration of solutes to the side with the higher concentration of solutes, in an atempt to dilute the solutes and equalize the concentration on both sides.
Osmosis is important in many biological processes, including the uptake of water by plant roots, the regulation of water balance in animal cells, and the preservation of food by adding salt or sugar to create a hypertonic environment that inhibits bacterial growth.
 |
Correct Answer is B
Explanation
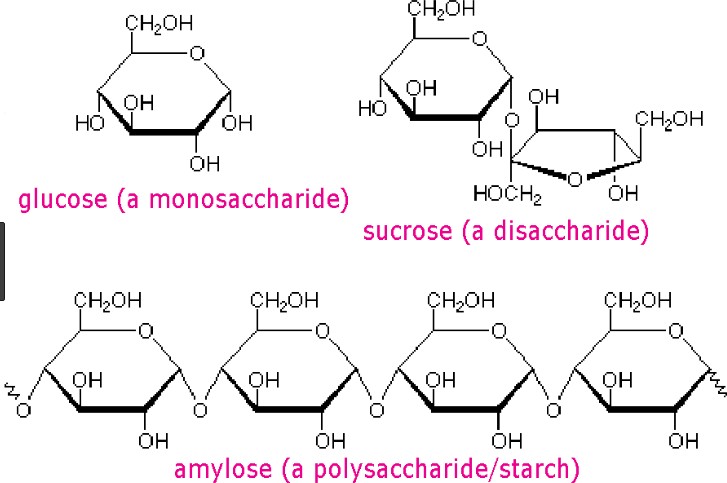
Carbohydrates are one of the main types of biomolecules and are composed of monomers called monosaccharides. Monosaccharides are simple sugars that cannot be further broken down into simpler sugars. They are usually composed of 3 to 7 carbon atoms and have a general formula of (CH2O)n, where n is a number between 3 and 7. Examples of monosaccharides include glucose, fructose, and galactose.
When two monosaccharides are joined together by a glycosidic bond, they form a disaccharide. Disaccharides are composed of two simple sugars and can be broken down into their constituent monosaccharides by hydrolysis. Examples of disaccharides include sucrose, lactose, and maltose.
Option a) is incorrect because it describes the composition of a disaccharide, not a monosaccharide. Option
c) is incorrect because both monosaccharides and disaccharides can be found in both plants and animals.
Option d) is incorrect because both monosaccharides and disaccharides can be used for energy storage and
structural purposes, depending on their specific structure and function in the organism.
 |
Correct Answer is C
Explanation
Stomach acid is highly acidic, primarily composed of hydrochloric acid (HCl), which means it has a low pH (around 1 to 3). Acids release hydrogen ions (H⁺) in solution, which lowers the pH.
- A. It has a higher pH: Incorrect, as acidic solutions have a lower pH compared to neutral distilled water (which has a pH of 7).
- B. It contains nitrogen: Incorrect, stomach acid is composed mostly of HCl, not nitrogen-containing compounds.
- D. It has more hydroxyl ions: Incorrect, acidic solutions have fewer hydroxyl ions (OH⁻); hydroxyl ions are more common in basic (alkaline) solutions.
In comparison to distilled water, which is neutral, the stomach acid solution has significantly more hydrogen ions, making it more acidic.
Correct Answer is B
Explanation
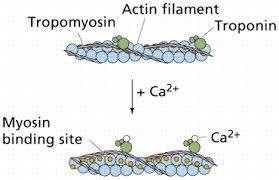
Muscle contraction is a complex process that involves the interaction between actin and myosin filaments in the muscle fibers. The sliding of these filaments is initiated by the release of calcium ions from the sarcoplasmic reticulum, a specialized organelle in muscle cells. The calcium ions bind to the protein troponin, which causes a conformational change in the troponin-tropomyosin complex, exposing the myosin-binding sites on actin. This allows the myosin heads to bind to actin, forming cross-bridges that pull the actin filaments towards the center of the sarcomere, resulting in muscle contraction.
Option a) is incorrect because calcium does not bind to tropomyosin directly, but rather binds to the protein troponin, causing a conformational change in the troponin-tropomyosin complex. Option c) is incorrect because calcium does not activate motor neurons, but rather is released from the sarcoplasmic reticulum in response to an action potential that travels down the motor neuron to the neuromuscular junction. Option d) is incorrect because calcium is required for muscle contraction, not relaxation. The relaxation of muscles after contraction is due to the active transport of calcium ions back into the sarcoplasmic reticulum, which allows the troponin-tropomyosin complex to return to its resting conformation, blocking the myosin-binding sites on actin and ending the cross-bridge cycle.
Correct Answer is B
Explanation
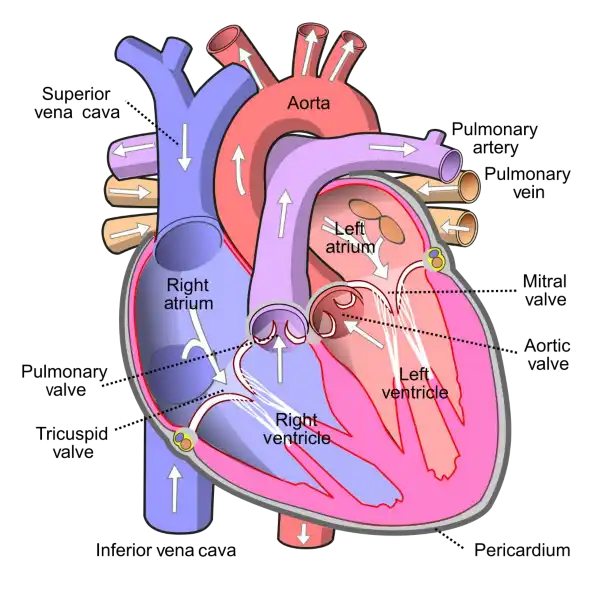
The mitral valve is located between the left atrium and left ventricle of the heart and helps to regulate the flow of blood between these chambers. It consists of two leaflets or flaps that open and close in response to changes in pressure as the heart beats.
During diastole, when the heart is relaxed and filling with blood, the mitral valve opens to allow blood to flow from the left atrium into the left ventricle. During systole, when the heart contracts to pump blood out of the left ventricle and into the systemic circulation, the mitral valve closes to prevent backflow of blood into the left atrium.
The mitral valve is one of four valves in the heart that help to ensure the unidirectional flow of blood through the heart and the rest of the circulatory system. Problems with the mitral valve, such as mitral valve prolapse or mitral stenosis, can lead to a range of symptoms and complications, including shortness of breath, fatigue, chest pain, and heart failure.
 |
Correct Answer is D
Explanation
This reaction involves an acid (hydrochloric acid, HCl) and a base (magnesium hydroxide, Mg(OH)₂) reacting to form water (H₂O) and a salt (magnesium chloride, MgCl₂). This is a classic neutralization reaction, where an acid reacts with a base to neutralize each other, producing water and a salt.
- Neutralization Reaction: Acid + Base → Water + Salt
- In this case:
- Acid: HCl (hydrochloric acid)
- Base: Mg(OH)₂ (magnesium hydroxide)
- Products: H₂O (water) and MgCl₂ (magnesium chloride)
The other options do not apply:
- A. Decomposition: A single compound breaks down into two or more substances. Not the case here.
- B. Combustion: A substance reacts with oxygen, often producing heat and light (usually with organic compounds). Not the case here.
- C. Synthesis: Two or more substances combine to form a single product. Not applicable to this reaction.
Correct Answer is D
Explanation
The unit used to indicate length is the meter (m). It is the base unit of length in the International System of Units (SI).
Correct Answer is C
Explanation
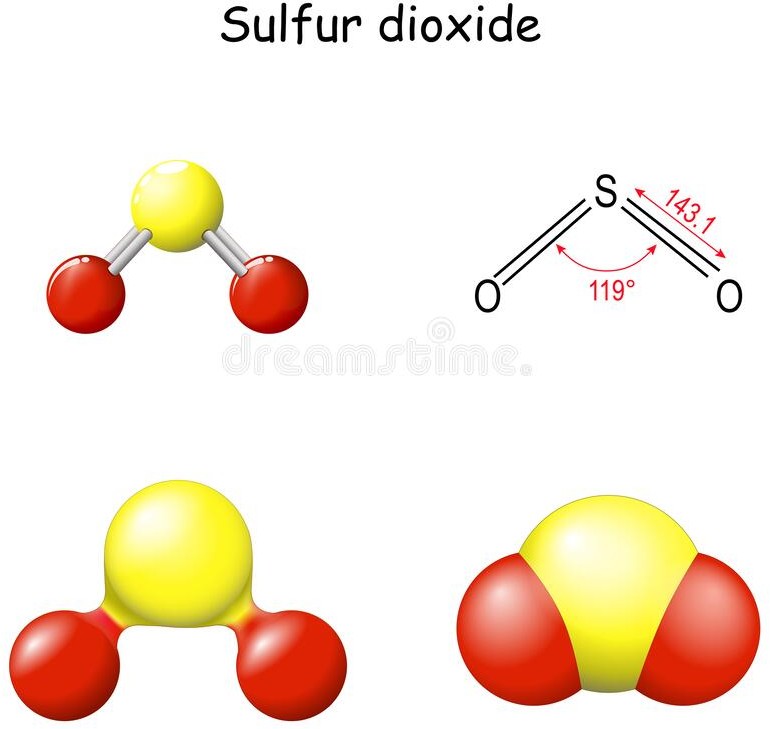
The molecular geometry of a molecule of sulphur dioxide (SO2) is bent or V-shaped. This is because of the presence of two lone pairs on the sulfur atom, which cause repulsion and distort the bond angles in the molecule.
SO2 has a central sulfur atom bonded to two oxygen atoms by double bonds. The two double bonds and the two lone pairs of electrons on sulfur result in a trigonal planar arrangement of electron pairs around the sulfur atom. However, the repulsion between the lone pairs causes the two oxygen atoms to be pulled closer together, resulting in a bent or V-shaped molecular geometry.
The bent molecular geometry of SO2 affects its properties, such as its polarity and reactivity. SO2 is a polar molecule due to the asymmetric distribution of electrons, which results in a partial positive charge on the sulfur atom and partial negative charges on the oxygen atoms. This polarity makes SO2 a good solvent and reactant in chemical reactions, as well as a contributor to air pollution and acid rain.
 |
This question was extracted from the actual TEAS Exam. Ace your TEAS exam with the actual TEAS 7 questions, Start your journey with us today
Visit Naxlex, the Most Trusted TEAS TEST Platform With Guaranteed Pass of 90%.
Money back guarantee if you use our service and fail the actual exam. Option of personalised live tutor on your area of weakness.
