What is the primary pigment responsible for photosynthesis in plants?
Chlorophyll a
Chlorophyll b
Carotenoids
Anthocyanins
Correct Answer : A
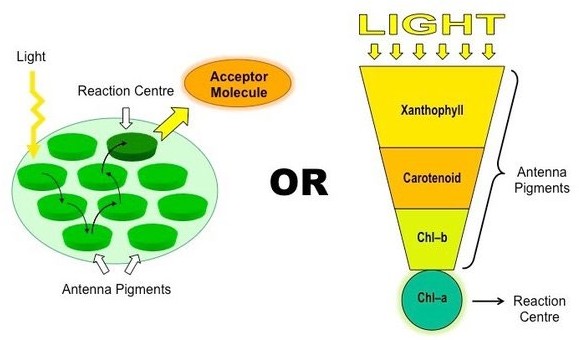
Chlorophyll a is the primary pigment responsible for photosynthesis in plants. It is a green pigment that is essential for capturing light energy from the sun and converting it into chemical energy that can be used by the plant. Chlorophyll a absorbs light most efficiently in the blue and red parts of the spectrum, and reflects green light, giving plants their characteristic green color
Chlorophyll b is another type of chlorophyll that is also involved in photosynthesis, but it is not as abundant as chlorophyll a. Chlorophyll b absorbs light most efficiently in the blue and orange parts of the spectrum and reflects yellow-green light.
Carotenoids are pigments that are present in many plants and are involved in photosynthesis as well as protecting the plant from damage caused by excess light. Carotenoids are responsible for the orange, yellow, and red colors of many fruits and vegetables.
Anthocyanins are pigments that give plants their red, purple, and blue colors. While they are not directly involved in photosynthesis, they play a role in atracting pollinators and protecting the plant from damage caused by UV radiation.
TEAS 7 Exam Quiz Bank
HESI A2 Exam Quiz Bank
Find More Questions 📚
Teas 7 Questions: We got the latest updated TEAS 7 questions
100% Money Refund: 100% money back guarantee if you take our full
assessment pass with 80% and fail the actual exam.
Live Tutoring: Fully customized live tutoring lessons.
Guaranteed A Grade: All students who use our services pass with 90%
guarantee.
Related Questions
Correct Answer is B
Explanation
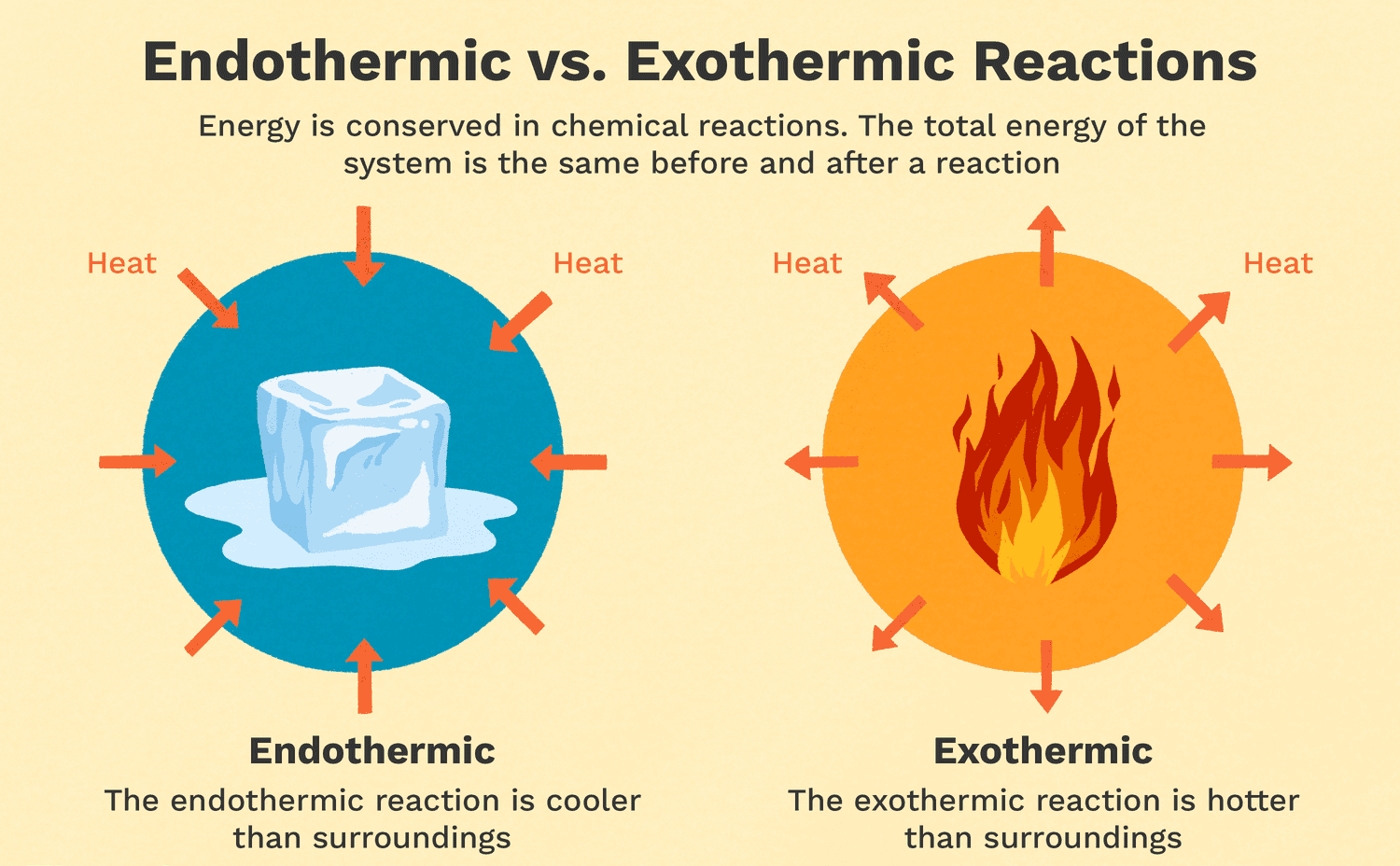
Exothermic reactions are reactions that release energy in the form of heat, light, or sound. Burning wood is an example of an exothermic reaction because it releases heat and light. As the wood reacts with oxygen in the air, it undergoes a combustion reaction that releases energy in the form of heat and light. Melting ice is an endothermic reaction because it requires energy input to melt the solid ice into liquid water. Cooking an egg is a chemical reaction that involves denaturing the proteins in the egg, but it is not necessarily exothermic or endothermic. Dissolving sugar in water is also not an example of an exothermic reaction because it does not release energy in the form of heat, light, or sound.
Correct Answer is C
Explanation
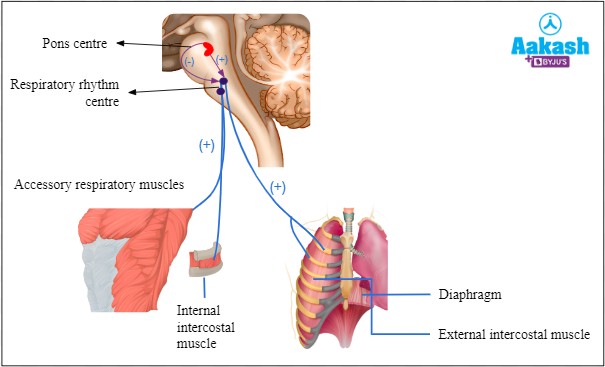
Diaphragm is responsible for regulating breathing rate and depth. It is a dome-shaped muscle located at the
bottom of the chest cavity that contracts and relaxes to help move air in and out of the lungs.
 |
Correct Answer is A
Explanation
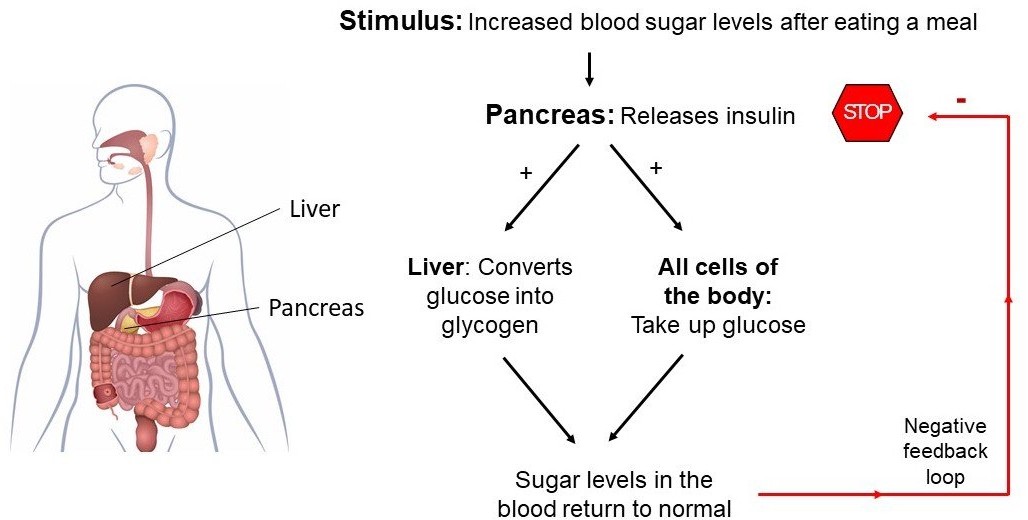
Insulin is a hormone produced by the pancreas that plays a crucial role in regulating the levels of glucose (sugar) in the blood. After a person eats a meal, the levels of glucose in the blood rise, which stimulates the pancreas to release insulin into the bloodstream. Insulin acts on various cells in the body, particularly those in the liver, muscles, and adipose tissue, to promote the uptake, use, and storage of glucose.
Insulin helps to lower the levels of glucose in the blood by increasing the uptake of glucose by cells, stimulating the liver and muscle cells to store glucose in the form of glycogen, and inhibiting the production and release of glucose by the liver. This process is known as glucose homeostasis, and it helps to keep the levels of glucose in the blood within a normal range.
Deficiencies or abnormalities in insulin production or function can lead to a range of metabolic disorders, including type 1 and type 2 diabetes. In type 1 diabetes, the body does not produce enough insulin, while in type 2 diabetes, the body becomes resistant to the effects of insulin, leading to elevated levels of glucose in the blood.
Correct Answer is C
Explanation
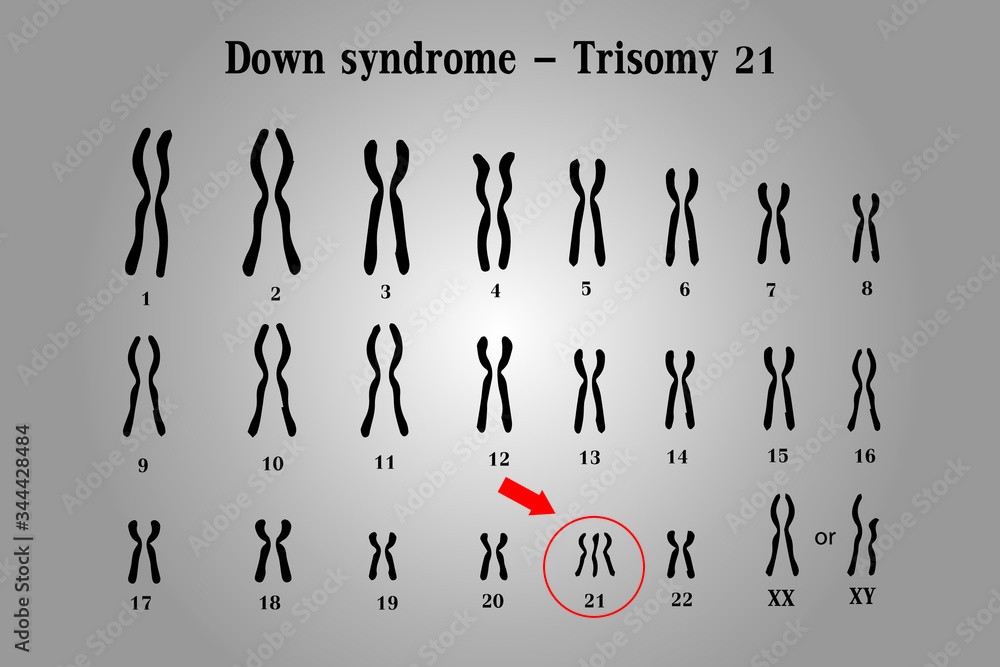
Down syndrome is a genetic disorder caused by the presence of an extra copy of chromosome 21. It is also known as trisomy 21, because affected individuals have three copies of chromosome 21 instead of the normal two.
The extra chromosome 21 in Down syndrome occurs due to a random error in cell division, which leads to the production of an abnormal gamete (egg or sperm) with an extra copy of the chromosome. When this gamete fuses with a normal gamete during fertilization, the resulting zygote has 47 chromosomes instead of the usual 46, and develops into a fetus with Down syndrome.
Down syndrome is characterized by a range of physical and intellectual symptoms, including developmental delays, intellectual disability, distinctive facial features, heart defects, and increased risk of certain medical conditions such as leukemia and Alzheimer's disease. However, the severity and expression of these symptoms can vary widely among affected individuals.
 |
Correct Answer is A
Explanation
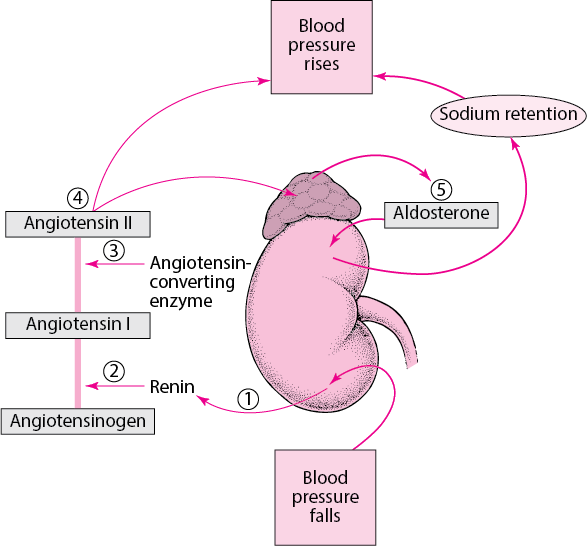
Renin is an enzyme that is produced by the kidneys and it acts to elevate blood pressure. When blood pressure falls, the kidneys secrete renin into the bloodstream ³.
 |
Correct Answer is B
Explanation
A buffer is a solution that resists changes in pH when small amounts of an acid or base are added. Buffers work by neutralizing added hydrogen ions (H⁺) or hydroxide ions (OH⁻), thereby maintaining a relatively stable pH. Buffers are made up of a weak acid and its conjugate base or a weak base and its conjugate acid.
- A. It decreases the pH of the solution: This is incorrect because a buffer does not always decrease pH; it resists changes in both directions.
- C. It causes the pH of a solution to become neutral: Incorrect because buffers do not necessarily make a solution neutral; they stabilize pH around a certain value.
- D. It permanently binds hydrogen ions: Incorrect as the binding is reversible, which is essential for maintaining pH balance.
Correct Answer is D
Explanation
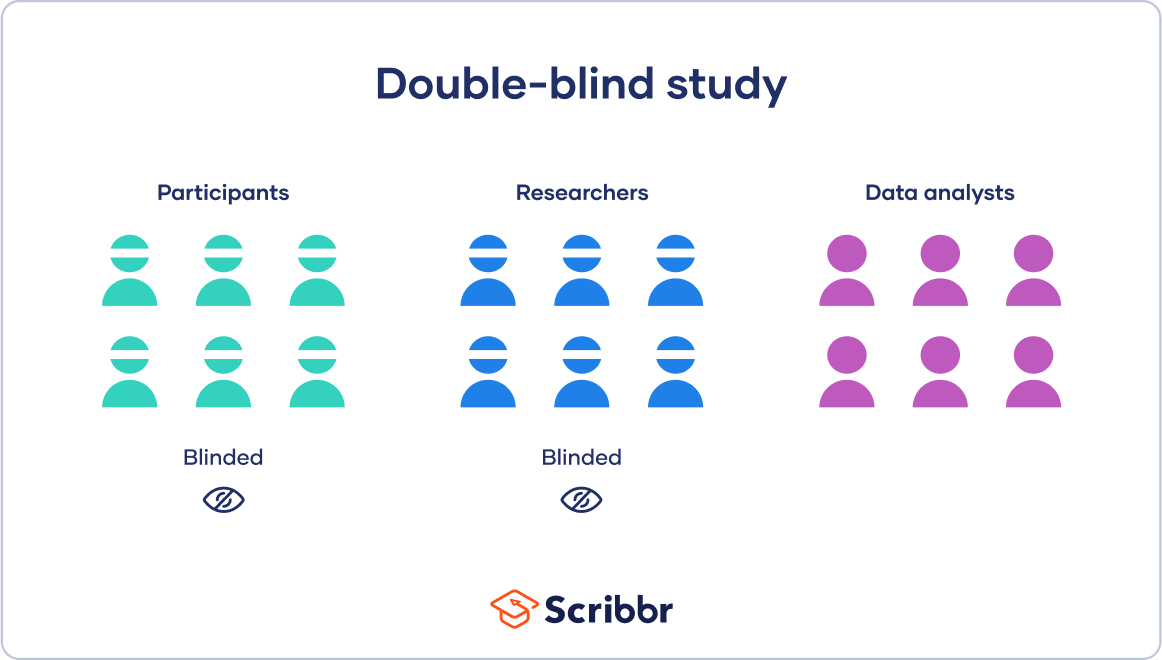
A double-blind study is a research design in which neither the participants nor the researchers know which group participants are assigned to. This is done to minimize bias and ensure that the results of the study are as objective as possible. In a double-blind study, the treatment and control groups are randomly assigned, and the participants and researchers are unaware of which group each participant is assigned to. Option a) is an example of a randomized controlled trial, which is a common research design, but it is not necessarily double-blind. Option b) is an example of an open-label study, in which both the participants and the researchers know which group each participant is assigned to. Option c) is an example of a single-blind study, in which the participants do not know which group they are assigned to, but the researchers do.
Correct Answer is A
Explanation
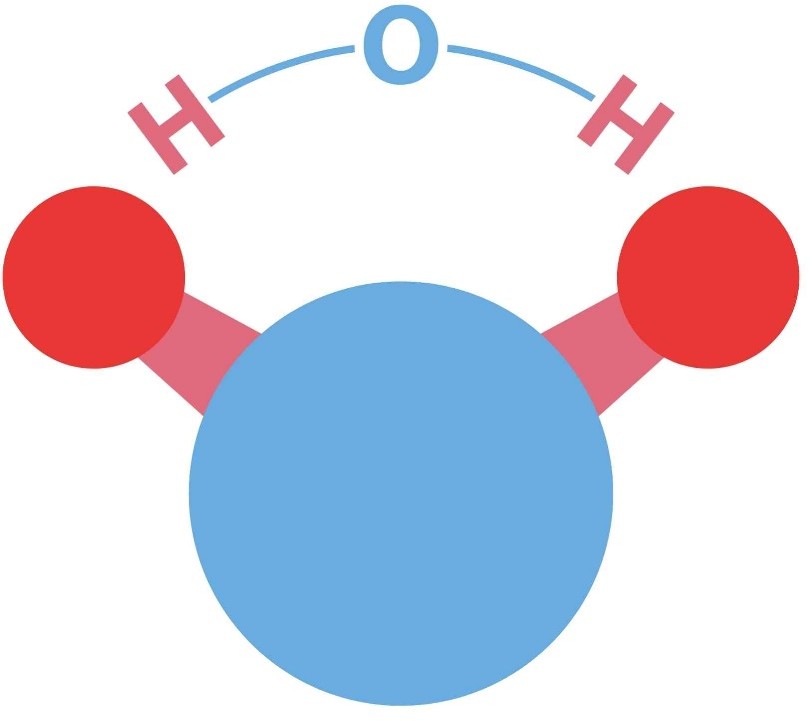
The chemical formula for water is H2O. It consists of two hydrogen atoms and one oxygen atom.
 |
Correct Answer is C
Explanation
Water molecules primarily enter cells through the process of facilitated diffusion, specifically via aquaporins, which are specialized channel proteins that facilitate the rapid transport of water across the cell membrane. This process does not require energy (ATP) as it relies on the concentration gradient of water, allowing water to move from areas of higher concentration to lower concentration.
Here’s why the other options are not correct in the context of water transport:
- A. Gated channels: While aquaporins can be gated, this term generally refers to channels that open and close in response to specific signals, which is not the primary mechanism for water transport in most cells.
- B. Electrochemical gradients: This term relates to the combined effect of electrical and chemical gradients across a membrane, typically for ions rather than water molecules directly. Water movement can be influenced by osmotic gradients but is not solely dependent on electrochemical gradients.
- D. Proton pumps: These are involved in transporting protons (H⁺ ions) across membranes, primarily for establishing an electrochemical gradient, not for the transport of water.
Thus, water molecules enter cells mainly by facilitated diffusion through aquaporins.
Correct Answer is A
Explanation
Transfer RNA (tRNA) is responsible for carrying amino acids to ribosomes during protein synthesis. Each tRNA molecule has a specific anticodon that matches a codon on the messenger RNA (mRNA) molecule. The tRNA molecule binds to the mRNA codon and brings the corresponding amino acid to the ribosome, where it is added to the growing polypeptide chain.
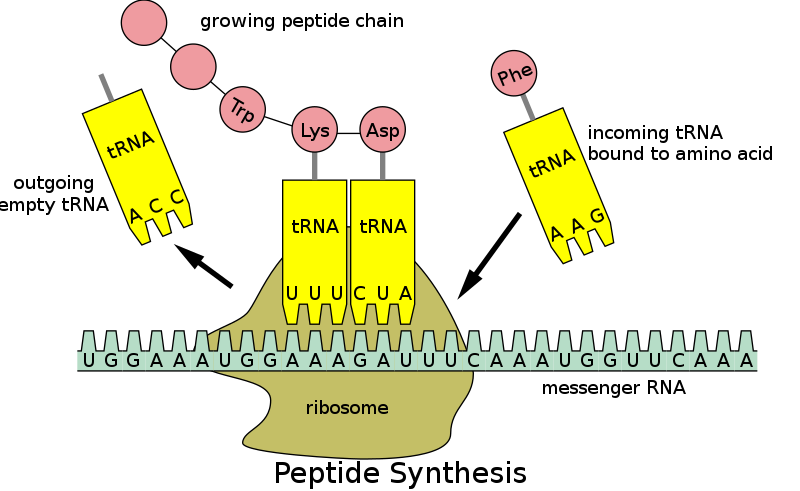 |
This question was extracted from the actual TEAS Exam. Ace your TEAS exam with the actual TEAS 7 questions, Start your journey with us today
Visit Naxlex, the Most Trusted TEAS TEST Platform With Guaranteed Pass of 90%.
Money back guarantee if you use our service and fail the actual exam. Option of personalised live tutor on your area of weakness.
