Which blood group is a universal donor?
A
B
AB
O
Correct Answer : D
A person can be a universal blood donor or acceptor. A universal blood donor has type O blood, while a universal blood acceptor has type AB blood.
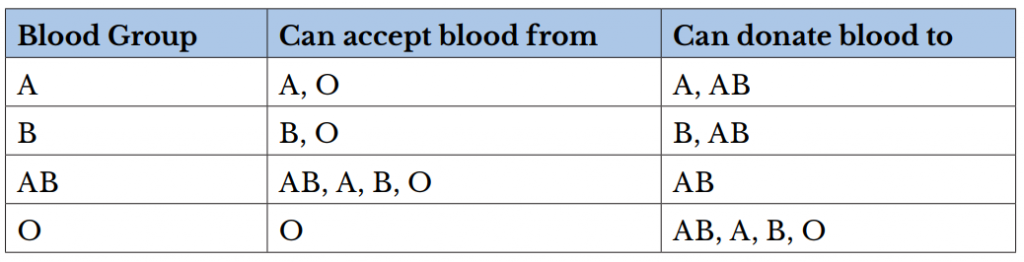
There are several different types or groups of blood, and the major groups are A, B, AB, and O. Blood group is a way to classify blood according to inherited differences of red blood cell antigens found on the surface of a red blood cell. The type of antibody in blood also identifies a particular blood group. Antibodies are proteins found in the plasma. They function as part of the body’s natural defense to recognize foreign substances and alert the immune system.
Depending on which antigen is inherited, parental offspring will have one of the four major blood groups. Collectively, the following major blood groups comprise the ABO system:
- Blood group A: Displays type A antigens on the surface of a red blood cell and contains B antibodies in the plasma.
- Blood group B: Displays type B antigens on the red blood cell’s surface and contains A antibodies in the plasma.
- Blood group O: Does not display A or B antigens on the surface of a red blood cell. Both A and B antibodies are in the plasma.
- Blood group AB: Displays type A and B antigens on the red blood cell’s surface, but neither A nor B antibodies are in the plasma
In addition to antigens, the Rh factor protein may exist on a red blood cell’s surface. Because this protein can be either present (+) or absent (-), it increases the number of major blood groups from four to eight: A+, A-, B+, B-, O+, O-, AB+, and AB-.
TEAS 7 Exam Quiz Bank
HESI A2 Exam Quiz Bank
Find More Questions 📚
Teas 7 Questions: We got the latest updated TEAS 7 questions
100% Money Refund: 100% money back guarantee if you take our full
assessment pass with 80% and fail the actual exam.
Live Tutoring: Fully customized live tutoring lessons.
Guaranteed A Grade: All students who use our services pass with 90%
guarantee.
Related Questions
Correct Answer is D
Explanation
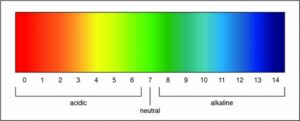
Both litmus paper and a pH scale can be used to indicate whether a solution is acidic. However, a pH scale can also determine the strength of an acid.
Researchers can determine the strength of an acid or a base by measuring the pH of a solution. The pH value describes how acidic or basic a solution is. On pH scale, shown below, if the number is less than 7 the solution is acidic. A pH greater than 7 means the solution is basic. When the pH is exactly 7, the solution is neutral.
Correct Answer is B
Explanation
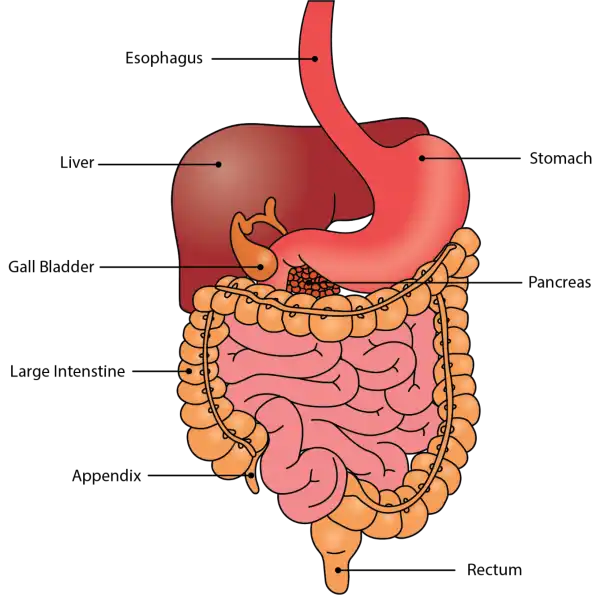
Oral Cavityis the first part of the digestive system. It is bounded by the lips and cheeks and contains the teeth and tongue. Its primary function is to masticate, or chew, and moisten the food.
Pharynx, or throat, connects the mouth to the esophagus.
Esophagusis a muscular tube about 25 centimeters long. Food travels down it to the cardiac sphincter of the stomach.
Pyloric sphincter. The exit of the stomach.
Small intestineis about 6 meters long and consists of three parts: duodenum, jejunum, and ileum.
Large intestine, consists of the cecum, colon, rectum, and anal canal. The cecum is located where the small and large intestine meet. The primary function of the large intestine is to compress the waste and collect any excess water that can be recycled.
Colonis about 1.5 to 1.8 meters long and consists of four parts: the ascending, transverse, descending, and sigmoid colon.
Correct Answer is A
Explanation
Robert Hooke discovered the first cells in the mid-eighteenth century. The cell theory is a theory because it is supported by a significant number of experimental findings. The cell theory took many years to be developed because microscopes were not powerful enough to make such observations.
This theory, or in-depth explanation, about cells consists of three parts:
- All living things are composed of one or more cells.
- Cells are alive and represent the basic unit of life.
- All cells are produced from pre-existing cells.
Correct Answer is A
Explanation
Atissueis a group of cells with similar structure and function and similar extracellular substances located between the cells. The table below describes the four primary tissues found in the human body.
body.
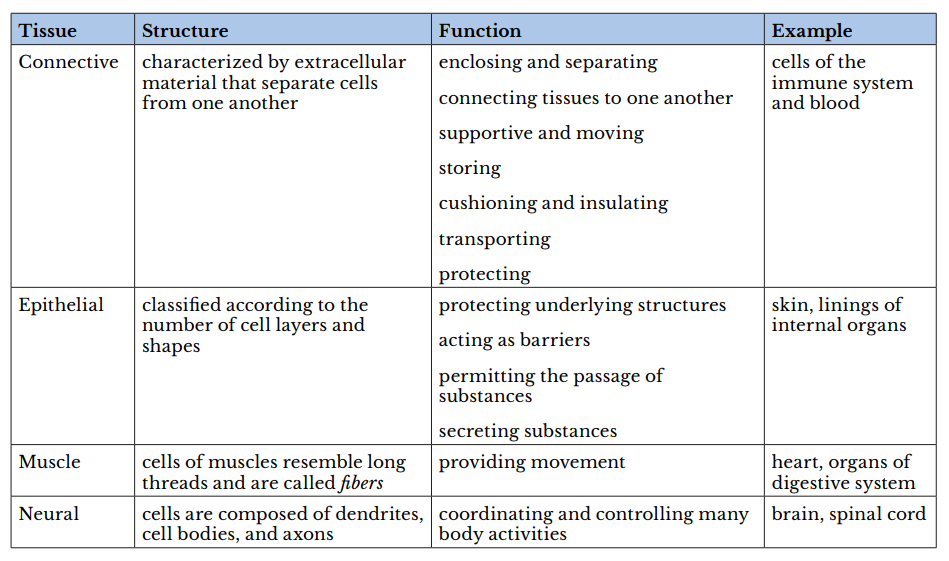
Correct Answer is D
Explanation
The primary function of the respiratory system is to provide oxygen to and remove carbon dioxide from the body. In addition to gas exchange, the respiratory system enables a person to breathe. Breathing, or inhalation, is essential to life. It is the mechanism that provides oxygen to the body. Without oxygen, cells are unable to perform their functions necessary to keep the body alive. The primary muscle of inspiration is the diaphragm. Known as the chest cavity, this dome shaped structure flattens when it contracts. The rib cage moves outward, allowing outside air to be drawn into the lungs. During relaxation, the diaphragm returns to its dome shape and the rib cage moves back to its natural position. This causes the chest cavity to push air out of the lungs.
The respiratory system can be functionally divided into two parts:
- Air-conducting portion: Air is delivered to the lungs. This region consists of the upper and lower respiratory tract—specifically, the larynx, trachea, bronchi, and bronchioles.
- Gas exchange portion: Gas exchange takes place between the air and the blood. This portion includes the lungs, alveoli, and capillaries.
Correct Answer is A
Explanation
The main male reproductive organs are thepenisand thetesticles, which are located external to the body. The penis is composed of a long shaft and a bulbous end called the glans penis. The glans penis is usually surrounded by an extension of skin called the foreskin.
Thetestes(analogous to the female ovaries), or testicles, are retained in a pouch of skin called thescrotum, which descends from the base of the penis. The scrotum contains nerves and blood vessels needed to support the testicles’ functions. Each testicle (or testis) produces sperm (analogous to the female ova), which are passed into a series of coiled tubules called theepididymis. The epididymis stores and nurtures sperm until they are passed into thevas deferens, a tubule that is about 30 centimeters long, extending from the testicle into the pelvis and ending at the ejaculatory duct.
The epididymis and vas deferens are supported by several accessory glands (the seminal vesicles, the prostate gland, and the Cowper glands) that produce fluid components of semen and support the sperm cells.
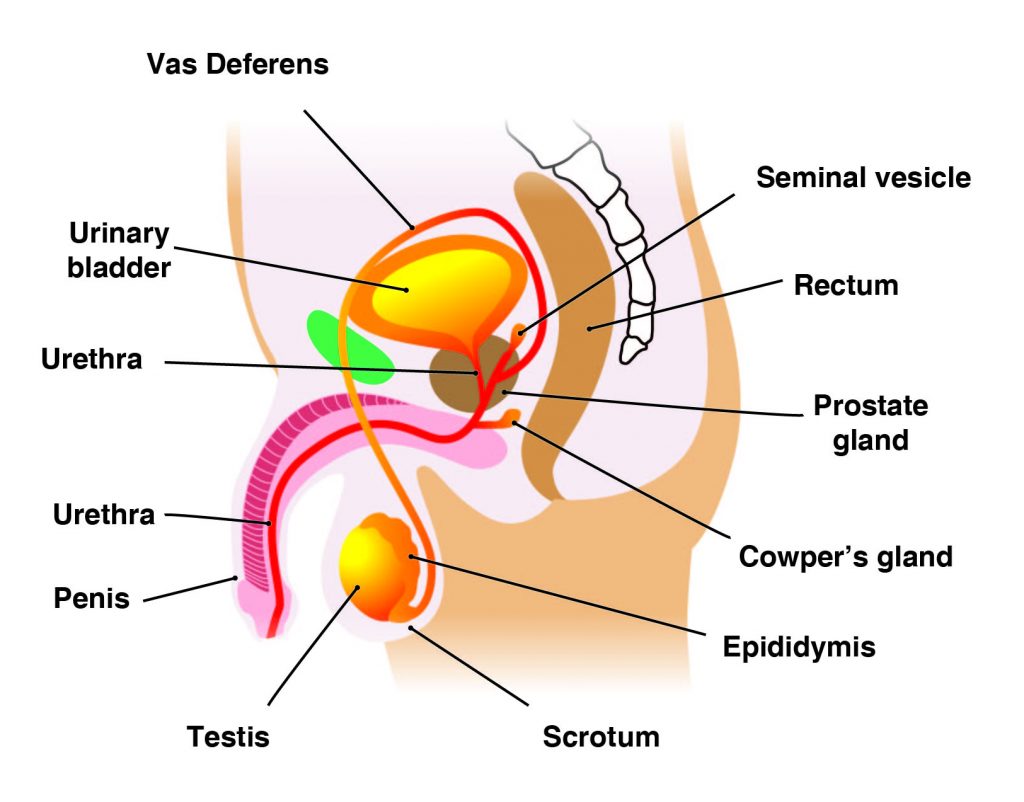
Correct Answer is D
Explanation
The cardiovascular system is closely associated with hematopoiesis because it includes the heart and blood vessels, which are responsible for circulating blood throughout the body. Hematopoiesis, the process of blood cell formation, primarily occurs in the bone marrow, which is part of the skeletal system. However, the cardiovascular system plays a crucial role in transporting these blood cells to various parts of the body once they are produced in the bone marrow.
So, while the skeletal system provides the site for hematopoiesis, the cardiovascular system is responsible for distributing the blood cells, making it the most closely associated system in this context.
Correct Answer is A
Explanation
The protein disc that holds two sister chromatids together is what collectively makes a chromosome. A gene is a segment of DNA, deoxyribonucleic acid, which transmits information from parent to offspring. A single molecule of DNA has thousands of genes. A chromosome is a rod-shaped structure that forms when a single DNA molecule and its associated proteins coil tightly before cell division.
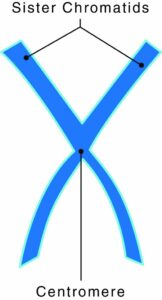
Chromosomes have two components:
- Chromatids: two copies of each chromosome
- Centromeres: protein discs that attach the chromatids together
Human cells have 23 sets of different chromosomes. The two copies of each chromosome are called homologous chromosomes, or homologues. An offspring receives one homologue from each parent. When a cell contains two homologues of each chromosome, it is termed diploid (2n). A haploid (n) cell contains only one homologue of each chromosome. The only haploid cells humans have are the sperm and eggs cells known as gametes.
Correct Answer is D
Explanation
Human intercourse consists of the male introducing sperm into the female’s reproductive system. Sperm may then pass through the female’s reproductive system to the Fallopian tubes where one sperm fertilizes an ovum, creating azygote. The zygote passes out of the Fallopian tube and implants into the uterine wall to begin gestation. Over nine months, the zygote develops and grows into an embryo and then a fetus. An infant is the baby that is born.
Correct Answer is D
Explanation
A sensory nerveis a nerve that carries sensory signals from the external environment to the brain to the central nervous system. It is also an afferent nerve, long dendrites of sensory neurons, which sends sensory information towards the central nervous system (CNS). This information is what is sensed, using the five senses from external environment, sight, sound, smell, taste, and touch.
Motor nerveshave onlyefferent fibers, long axons of motor neurons, that carry impulses away from the CNS to the effectors, which are typically tissues and muscles of the body.
Interneuronsarenerve cellsthat act as a bridge between motor and sensory neurons in the CNS. These neurons help form neural circuits, which helps neurons communicate with each other.
This question was extracted from the actual TEAS Exam. Ace your TEAS exam with the actual TEAS 7 questions, Start your journey with us today
Visit Naxlex, the Most Trusted TEAS TEST Platform With Guaranteed Pass of 90%.
Money back guarantee if you use our service and fail the actual exam. Option of personalised live tutor on your area of weakness.
