Which of the following determines the strength of an acidic solution?
Litmus paper that turns red
Litmus paper that turns blue
Measured pH value equal to 7
Measured pH value less than 7
Correct Answer : D
Both litmus paper and a pH scale can be used to indicate whether a solution is acidic. However, a pH scale can also determine the strength of an acid.
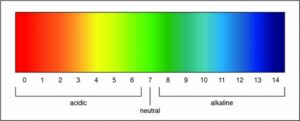
Researchers can determine the strength of an acid or a base by measuring the pH of a solution. The pH value describes how acidic or basic a solution is. On pH scale, shown below, if the number is less than 7 the solution is acidic. A pH greater than 7 means the solution is basic. When the pH is exactly 7, the solution is neutral.
TEAS 7 Exam Quiz Bank
HESI A2 Exam Quiz Bank
Find More Questions 📚
Teas 7 Questions: We got the latest updated TEAS 7 questions
100% Money Refund: 100% money back guarantee if you take our full
assessment pass with 80% and fail the actual exam.
Live Tutoring: Fully customized live tutoring lessons.
Guaranteed A Grade: All students who use our services pass with 90%
guarantee.
Related Questions
Correct Answer is B
Explanation
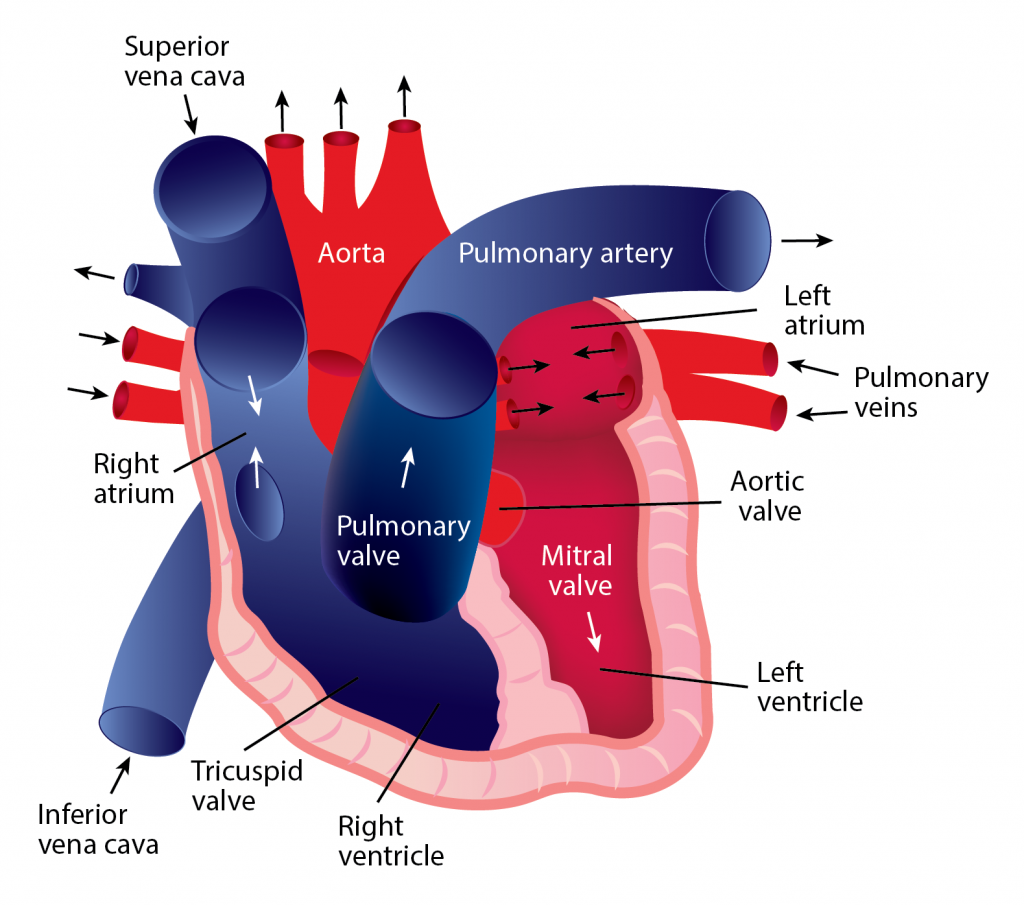
Blood continually flows in one direction, beginning in the heart and proceeding to the arteries, arterioles, and capillaries. When blood reaches the capillaries, exchanges occur between blood and tissues. After this exchange happens, blood is collected into venules, which feed into veins and eventually flow back to the heart’s atrium. The heart must relax between two heartbeats for blood circulation to begin.
Two types of circulatory processes occur in the body:
Systemic circulation
- The pulmonary vein pushes oxygenated blood into the left atrium.
- As the atrium relaxes, oxygenated blood drains into the left ventricle through the mitral valve. 3. The left ventricle pumps oxygenated blood to the aorta.
- Blood travels through the arteries and arterioles before reaching the capillaries that surround the tissues.
Pulmonary circulation
- Two major veins, the Superior Vena Cava and the Inferior Vena Cava, brings deoxygenated blood from the upper and lower half of the body.
- Deoxygenated blood is pooled into the right atrium and then sent into the right ventricle through the tricuspid valve, which prevents blood from flowing backward.
- The right ventricle contracts, causing the blood to be pushed through the pulmonary valve into the pulmonary artery.
- Deoxygenated blood becomes oxygenated in the lungs.
- Oxygenated blood returns from the lungs to the left atrium through the pulmonary veins.
Correct Answer is D
Explanation
The primary function of the respiratory system is to provide oxygen to and remove carbon dioxide from the body. In addition to gas exchange, the respiratory system enables a person to breathe. Breathing, or inhalation, is essential to life. It is the mechanism that provides oxygen to the body. Without oxygen, cells are unable to perform their functions necessary to keep the body alive. The primary muscle of inspiration is the diaphragm. Known as the chest cavity, this dome shaped structure flattens when it contracts. The rib cage moves outward, allowing outside air to be drawn into the lungs. During relaxation, the diaphragm returns to its dome shape and the rib cage moves back to its natural position. This causes the chest cavity to push air out of the lungs.
The respiratory system can be functionally divided into two parts:
- Air-conducting portion: Air is delivered to the lungs. This region consists of the upper and lower respiratory tract—specifically, the larynx, trachea, bronchi, and bronchioles.
- Gas exchange portion: Gas exchange takes place between the air and the blood. This portion includes the lungs, alveoli, and capillaries.
Correct Answer is A
Explanation
The cell cycle is an organized process divided into two phases:interphaseand theM (mitotic) phase. During interphase, the cell grows and copies its DNA. After the cell reaches the M phase, division of the two new cells can occur. The G1, S, and G2phases make up interphase.
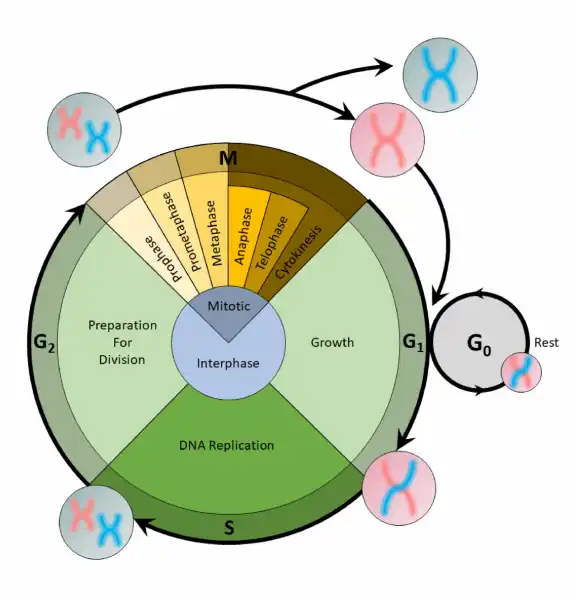
- G1:The first gap phase, during which the cell prepares to copy its DNA
- S:The synthesis phase, during which DNA is copied
- G2:The second gap phase, during which the cell prepares for cell division
It may appear that little is happening in the cell during the gap phases. Most of the activity occurs at the level of enzymes and macromolecules. The cell produces things like nucleotidesfor synthesizing new DNA strands, enzymes for copying the DNA, and tubulin proteins for building the mitotic spindle. During the S phase, the DNA in the cell doubles, but few other signs are obvious under the microscope. All the dramatic events that can be seen under a microscope occur during the M phase: the chromosomes move, and the cell splits into two new cells with identical nuclei.
Correct Answer is B
Explanation
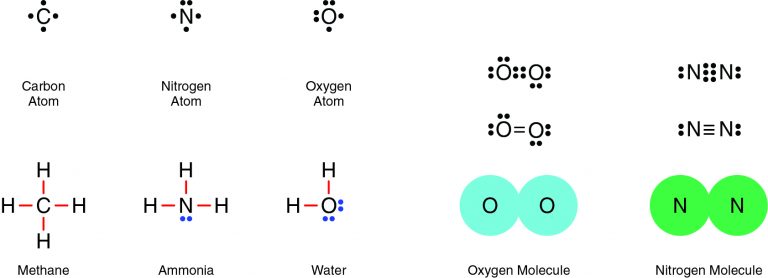
Nitrogen and oxygen are both nonmetals, which means they will share electrons in a covalent bond. For example, two oxygen atoms form a double bond, in which two pairs of electrons (four electrons total) are shared. Similarly, two nitrogen atoms form a molecule with a triple bond, in which three pairs of electrons (six electrons total) are shared.
Correct Answer is B
Explanation
In this reaction, chlorine (Cl2) is an element in the reaction that replaces iodine in the compound sodium iodide (NaI). This allows chlorine to form a compound with sodium (NaCl) and leaves iodine (I2) as an element.
Synthesisreactions involve two or more reactants (A and B) combining to form one product (AB). In the example provided, hydrogen (H2) and oxygen (O2) begin as separate elements. At the end of the reaction, the hydrogen and oxygen atoms are bonded in a molecule of water (H2O).
Decompositionreactions have only one reactant (AB) that breaks apart into two or more products (A and B). In the example above, hydrogen peroxide (H2O2) breaks apart into two smaller molecules: water (H2O) and oxygen (O2).
Single-replacementreactions involve two reactants, one compound (AB) and one element (C). In this type of reaction, one element replaces another to form a new compound (AC), leaving one element by itself (B). In the example, zinc replaces hydrogen in hydrochloric acid (HCl). As a result, zinc forms a compound with chlorine, zinc chloride (ZnCl2), and hydrogen (H2) is left by itself.
Double-replacementreactions involve two reactants, both of which are compounds made of two components (AB and CD). In the example, silver nitrate, composed of silver (Ag1+) and nitrate (NO31-) ions, reacts with sodium chloride, composed of sodium (Na1+) and chloride (Cl1-) ions. The nitrate and chloride ions switch places to produce two compounds that are different from those in the reactants.
Combustionreactions occur when fuels burn, and they involve specific reactants and products, as seen in the examples below. Some form of fuel that contains carbon and hydrogen is required. Examples of such fuels are methane, propane in a gas grill, butane in a lighter, and octane in gasoline. Notice that these fuels all react with oxygen, which is necessary for anything to burn. In all combustion reactions, carbon dioxide, water, and energy are produced. When something burns, energy is released, which can be felt as heat and seen as light.
Correct Answer is D
Explanation
The autonomic nervous systemis responsible for activities that arenonvoluntaryand under unconscious control. This system controls glands and the smooth muscles of internal organs, heart rate, breathing, and digestion. The autonomic nervous system is further divided into the following:
- Sympathetic nervous system: The sympathetic nervous system focuses on emergency situations by preparing the body forfight or flight. (Sympathetic = Stress)
- Parasympathetic nervous system: The parasympathetic nervous system controls involuntary processes unrelated to emergencies. This system deals with “rest or digest” activities. (Parasympathetic = Peace)
Thesomatic nervous systemprimarily controlsvoluntaryactivities such as walking and riding a bicycle. Thus, this system sends information to the CNS and motor nerve fibers that are attached to skeletal muscle.
Correct Answer is B
Explanation
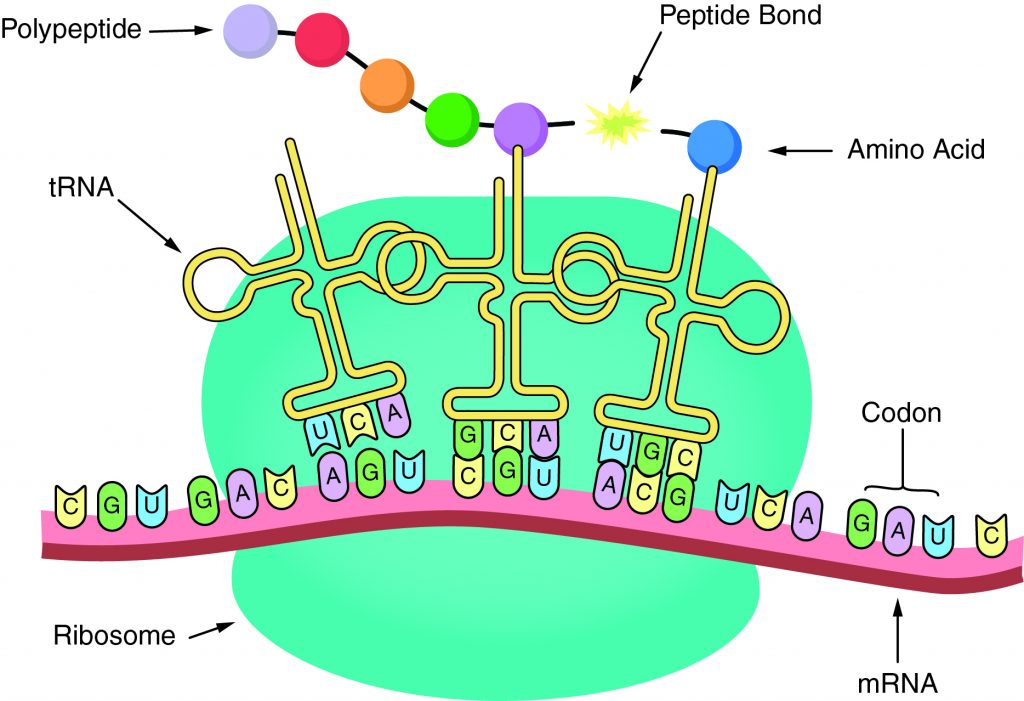
The sequence of amino acids in a gene determines the primary structure of a protein. The components necessary fortranslationare located in the cytoplasm. Translation is the making of proteins by mRNA binding to a ribosome with the start codon that initiates the production of amino acids. Apeptide bondforms and connects the amino acids together. The sequence of amino acids determines the protein’s structure, which determines its function.
Correct Answer is B
Explanation
The phenotype is the physical appearance of an organism, and the genotype is the set of alleles.
Mendel’s Theory of Heredity
To explain his results, Mendel proposed a theory that has become the foundation of the science of genetics. The theory has five elements:
- Parents do not transmit traits directly to their offspring. Rather, they pass on units of information calledgenes.
- For each trait, an individual has two factors: one from each parent. If the two factors have the same information, the individual ishomozygousfor that trait. If the two factors are different, the individual isheterozygousfor that trait. Each copy of a factor, orgene, is called anallele.
- The alleles determine the physical appearance, orphenotype. The set of alleles an individual has is itsgenotype.
- An individual receives one allele from each parent.
- The presence of an allele does not guarantee that the trait will be expressed
Correct Answer is B
Explanation
Mendel was accurately able to predict the patterns of heredity by studying rules related to genetics. These rules helped shape his theory of heredity. Heredity is the characteristics offspring inherit from their parents.
From experiments with garden peas, Mendel developed a simple set of rules that accurately predicted patterns of heredity. He discovered that plants eitherself-pollinateorcross-pollinate, when the pollen from one plant fertilizes the pistil of another plant. He also discovered that traits are eitherdominantorrecessive. Dominant traits are expressed, and recessive traits are hidden.
Mendel’s Theory of Heredity
To explain his results, Mendel proposed a theory that has become the foundation of the science of genetics. The theory has five elements:
- Parents do not transmit traits directly to their offspring. Rather, they pass on units of information calledgenes.
- For each trait, an individual has two factors: one from each parent. If the two factors have the same information, the individual ishomozygousfor that trait. If the two factors are different, the individual isheterozygousfor that trait. Each copy of a factor, orgene, is called anallele.
- The alleles determine the physical appearance, orphenotype. The set of alleles an individual has is itsgenotype.
- An individual receives one allele from each parent.
- The presence of an allele does not guarantee that the trait will be expressed.
Correct Answer is D
Explanation
A sensory nerveis a nerve that carries sensory signals from the external environment to the brain to the central nervous system. It is also an afferent nerve, long dendrites of sensory neurons, which sends sensory information towards the central nervous system (CNS). This information is what is sensed, using the five senses from external environment, sight, sound, smell, taste, and touch.
Motor nerveshave onlyefferent fibers, long axons of motor neurons, that carry impulses away from the CNS to the effectors, which are typically tissues and muscles of the body.
Interneuronsarenerve cellsthat act as a bridge between motor and sensory neurons in the CNS. These neurons help form neural circuits, which helps neurons communicate with each other.
This question was extracted from the actual TEAS Exam. Ace your TEAS exam with the actual TEAS 7 questions, Start your journey with us today
Visit Naxlex, the Most Trusted TEAS TEST Platform With Guaranteed Pass of 90%.
Money back guarantee if you use our service and fail the actual exam. Option of personalised live tutor on your area of weakness.
