Which of the following is an example of an exothermic reaction?
Melting ice
Burning wood
Cooking an egg
Dissolving sugar in water
Correct Answer : B
Exothermic reactions are reactions that release energy in the form of heat, light, or sound. Burning wood is an example of an exothermic reaction because it releases heat and light. As the wood reacts with oxygen in the air, it undergoes a combustion reaction that releases energy in the form of heat and light. Melting ice is an endothermic reaction because it requires energy input to melt the solid ice into liquid water. Cooking an egg is a chemical reaction that involves denaturing the proteins in the egg, but it is not necessarily exothermic or endothermic. Dissolving sugar in water is also not an example of an exothermic reaction because it does not release energy in the form of heat, light, or sound.
TEAS 7 Exam Quiz Bank
HESI A2 Exam Quiz Bank
Find More Questions 📚
Teas 7 Questions: We got the latest updated TEAS 7 questions
100% Money Refund: 100% money back guarantee if you take our full
assessment pass with 80% and fail the actual exam.
Live Tutoring: Fully customized live tutoring lessons.
Guaranteed A Grade: All students who use our services pass with 90%
guarantee.
Related Questions
Correct Answer is B
Explanation
Transcription is the process by which DNA is copied into RNA. During transcription, the DNA molecule unwinds and RNA polymerase reads the DNA sequence and synthesizes a complementary RNA molecule using the DNA as a template.
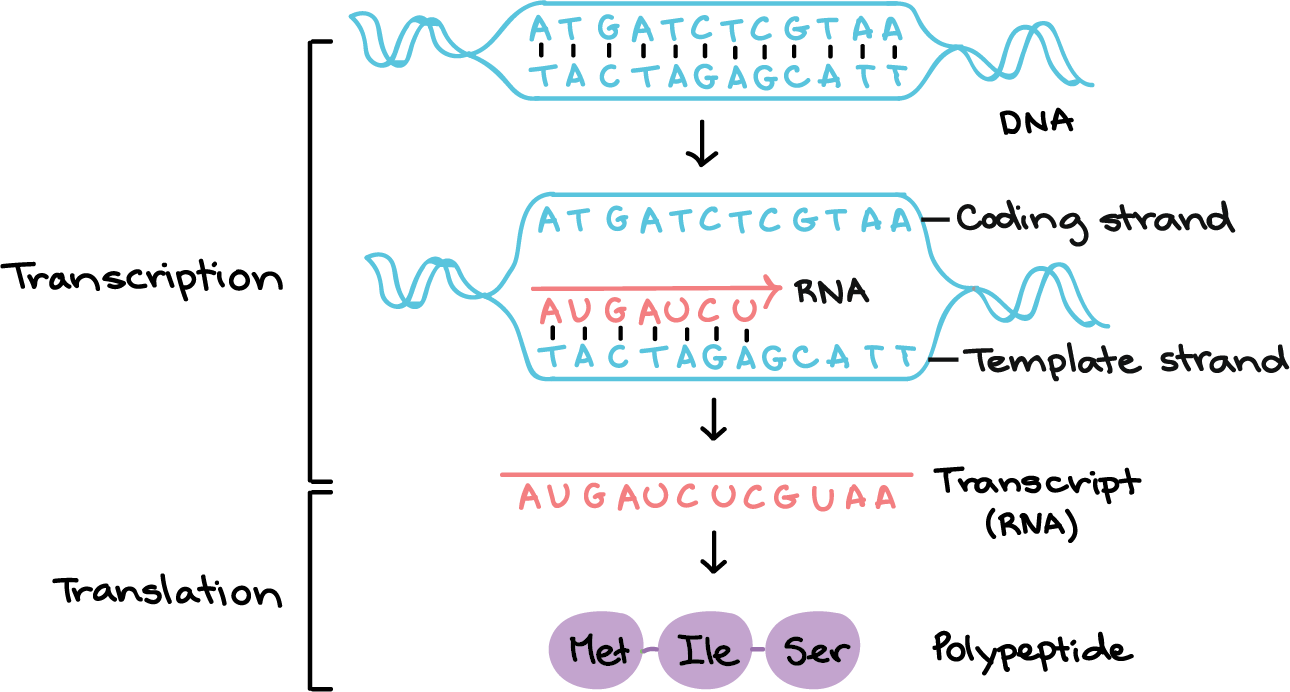
Correct Answer is C
Explanation
Facial acne is commonly caused by the blockage and inflammation of the sebaceous glands. These glands are responsible for secreting sebum, an oily substance that helps to lubricate the skin and hair. When these glands become overactive, or their ducts are blocked by excess sebum and dead skin cells, it can lead to the formation of pimples, blackheads, or whiteheads, which are typical signs of acne.
- Sebaceous glands are found near hair follicles and are primarily responsible for acne when they become clogged.
The other options are not related to acne:
- A. Lacrimal glands: These produce tears and are associated with the eye, not the skin.
- B. Sudoriferous glands: These are sweat glands, which are involved in perspiration, not typically linked to acne.
- D. Ceruminous glands: These are found in the ear canal and produce earwax, not involved in facial skin health.
Correct Answer is C
Explanation
One of the main functions of the respiratory system is to facilitate the exchange of gases between the body and the environment. During inhalation, air enters the lungs and oxygen is absorbed into the bloodstream. During exhalation, carbon dioxide is removed from the body and expelled into the environment.
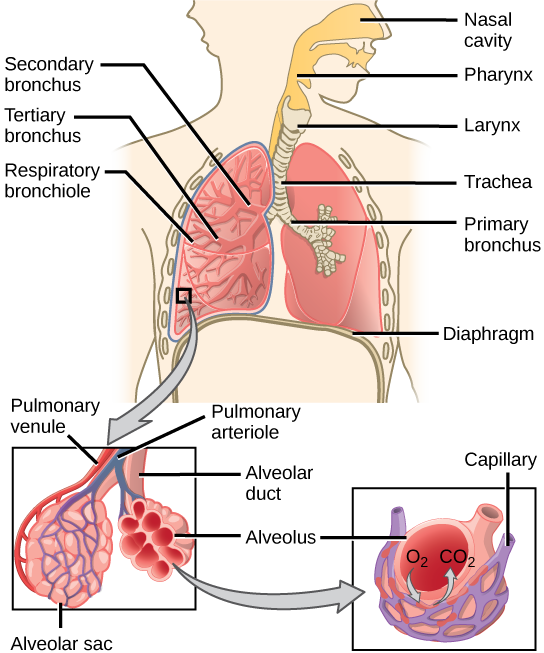 |
Correct Answer is B
Explanation
The perineum is the region of the body located between the pubic symphysis (in the front) and the coccyx (in the back). It includes the areas surrounding the openings for the urinary, digestive, and reproductive systems, such as the urethra, anus, and, in females, the vaginal opening.
The other options describe different areas of the body:
- A. The area on the back between the neck and the two shoulder blades refers to the upper back and shoulder region, not the perineum.
- C. The area between the nipples on the chest and the belly button describes the mid-torso, not the perineum.
- D. The area between the edges of the eyes and the chin describes the face, not the perineum.
Correct Answer is A
Explanation
The scientific method is a systematic approach used to answer questions or test hypotheses about the natural world. The steps involved in the scientific method are:
- Observation: This is the first step in the scientific method. It involves observing a phenomenon or a problem and gathering information about it.
- Hypothesis: After making an observation, a scientist forms a hypothesis, which is a tentative explanation for the phenomenon or problem.
- Prediction: Based on the hypothesis, the scientist makes a prediction about what will happen in an experiment or what they will observe.
- Experimentation: The scientist designs and conducts an experiment to test the hypothesis and prediction.
- Analysis: The data collected from the experiment are analyzed to determine if they support or refute the hypothesis.
- Conclusion: Based on the analysis of the data, the scientist draws a conclusion about whether the hypothesis is supported or refuted.
Option b) is incorrect because it starts with hypothesis before observation. Option c) is incorrect because prediction comes before experimentation. Option d) is incorrect because hypothesis comes after observation and data collection.
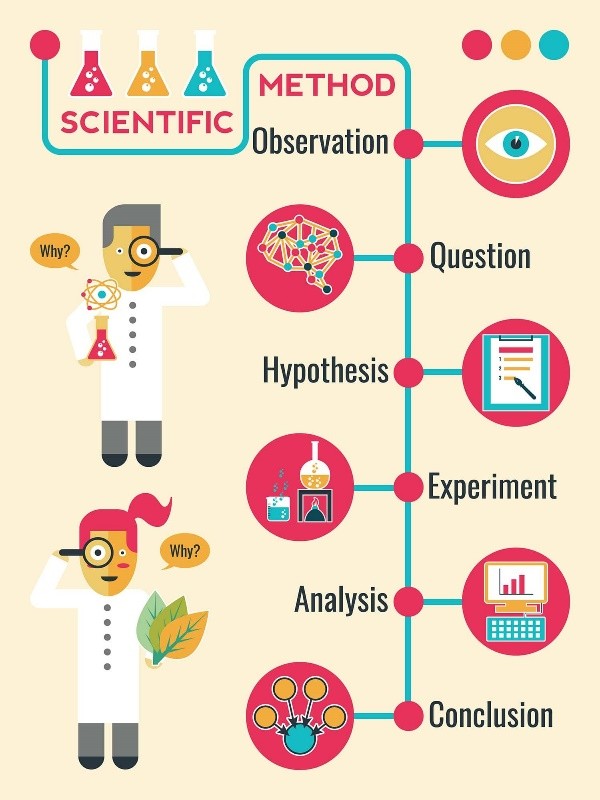 |
Correct Answer is C
Explanation
Water molecules primarily enter cells through the process of facilitated diffusion, specifically via aquaporins, which are specialized channel proteins that facilitate the rapid transport of water across the cell membrane. This process does not require energy (ATP) as it relies on the concentration gradient of water, allowing water to move from areas of higher concentration to lower concentration.
Here’s why the other options are not correct in the context of water transport:
- A. Gated channels: While aquaporins can be gated, this term generally refers to channels that open and close in response to specific signals, which is not the primary mechanism for water transport in most cells.
- B. Electrochemical gradients: This term relates to the combined effect of electrical and chemical gradients across a membrane, typically for ions rather than water molecules directly. Water movement can be influenced by osmotic gradients but is not solely dependent on electrochemical gradients.
- D. Proton pumps: These are involved in transporting protons (H⁺ ions) across membranes, primarily for establishing an electrochemical gradient, not for the transport of water.
Thus, water molecules enter cells mainly by facilitated diffusion through aquaporins.
Correct Answer is A
Explanation
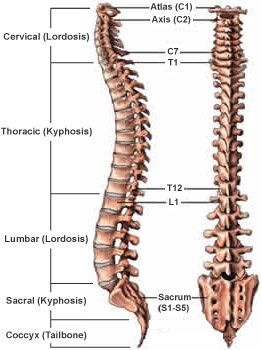
The vertebral column, also known as the spine or spinal column, is a series of bones called vertebrae that extend from the skull to the pelvis. It provides support for the body and protects the spinal cord. The five regions of the vertebral column, starting from the top and moving downwards, are:
- Cervical: This region is made up of seven vertebrae and is located in the neck. The first two cervical vertebrae, the atlas and the axis, are specialized to allow for head movement.
- Thoracic: This region is made up of twelve vertebrae and is located in the upper and middle back. The thoracic vertebrae are larger than the cervical vertebrae and articulate with the ribs.
- Lumbar: This region is made up of five vertebrae and is located in the lower back. The lumbar vertebrae are the largest and strongest of the vertebrae.
- Sacral: This region is made up of five fused vertebrae and is located in the pelvis. The sacrum forms the posterior wall of the pelvis and articulates with the hip bones.
- Coccygeal: This region is made up of four fused vertebrae and is located at the base of the vertebral column. The coccyx, or tailbone, provides atachment points for muscles and ligaments.
 |
Correct Answer is D
Explanation
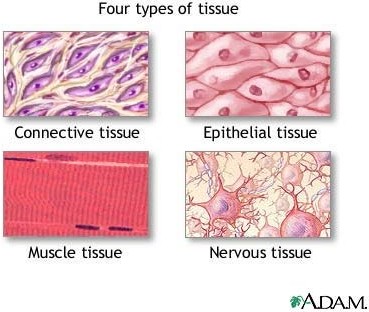
Exocrine glandular is not one of the four primary tissue types found in the human body. The four primary tissue types are epithelial, nervous, connective, and muscle.
 |
Correct Answer is C
Explanation
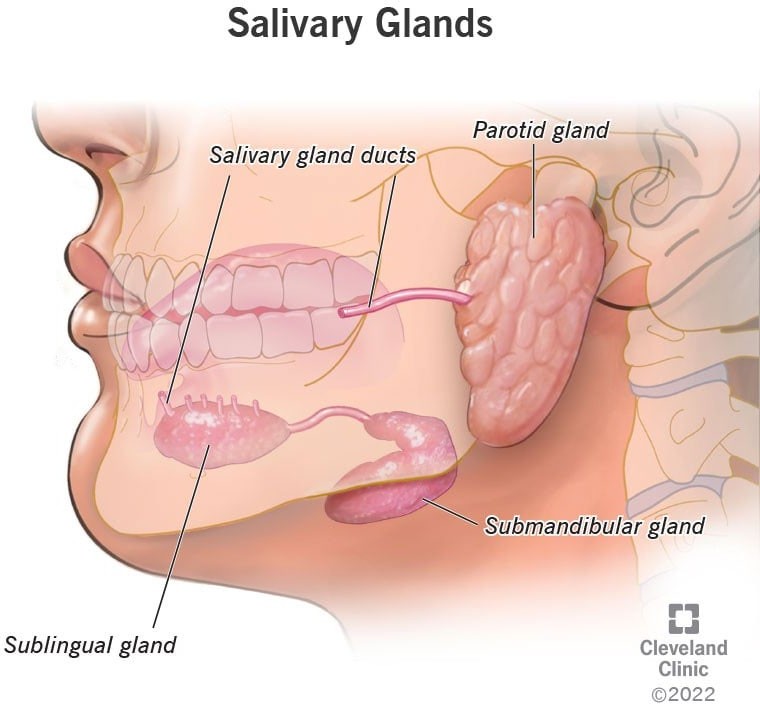
The three major pairs of salivary glands are the parotid glands, sublingual glands, and submandibular glands.
- Parotid glands are located just in front of your ears.
- Sublingual glands are located below either side of your tongue, under the floor of your mouth.
- Submandibular glands are located below your jaw.
 |
Correct Answer is D
Explanation
In the case of methanol poisoning, the metabolism of methanol to formaldehyde is a critical concern because formaldehyde is highly toxic. Ethanol is used as a treatment because it competes with methanol for the same enzyme, methanol oxidase (or alcohol dehydrogenase), effectively inhibiting the metabolism of methanol. By inhibiting the enzyme, ethanol reduces the conversion of methanol to formaldehyde, thereby minimizing its toxic effects.
Here’s why the other options are not suitable treatments:
- A. Methanol oxidase, which would increase the rate of the reaction: This would not be a treatment; it would worsen the situation by promoting the conversion of methanol to toxic formaldehyde.
- B. Methanol, which would saturate the methanol oxidase: This option would also be harmful, as adding more methanol would only lead to more formaldehyde production.
- C. Ice, which would shift the equilibrium of the reaction: The reaction is not a typical equilibrium reaction in this context, and cooling the body does not address the metabolic conversion of methanol to formaldehyde.
Thus, administering ethanol is an effective treatment to prevent the toxic effects of methanol metabolism.
This question was extracted from the actual TEAS Exam. Ace your TEAS exam with the actual TEAS 7 questions, Start your journey with us today
Visit Naxlex, the Most Trusted TEAS TEST Platform With Guaranteed Pass of 90%.
Money back guarantee if you use our service and fail the actual exam. Option of personalised live tutor on your area of weakness.
