Which of the following is the number of protons in a lithium atom?
7
3
12
4
Correct Answer : B
The atomic number of an element represents the number of protons in the nucleus of an atom of that element.
Since lithium has an atomic number of 3, it has 3 protons in its nucleus.
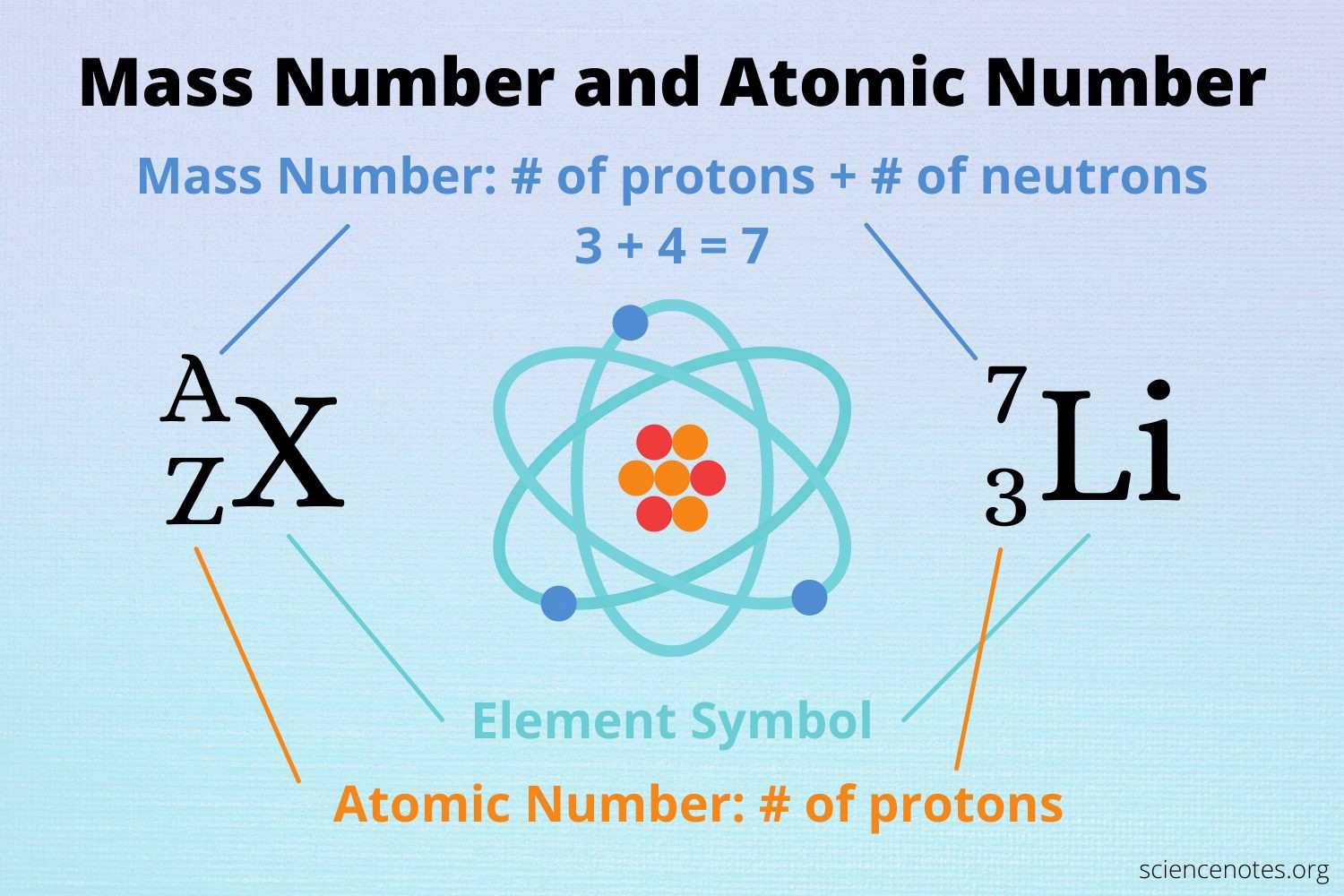
Choice A is not correct because 7 is the mass number of lithium, not the number of protons.
Choice C is not correct because 12 is not the atomic number or mass number of lithium.
Choice D is not correct because 4 is not the atomic number or mass number of lithium.
TEAS 7 Exam Quiz Bank
HESI A2 Exam Quiz Bank
Find More Questions 📚
Teas 7 Questions: We got the latest updated TEAS 7 questions
100% Money Refund: 100% money back guarantee if you take our full
assessment pass with 80% and fail the actual exam.
Live Tutoring: Fully customized live tutoring lessons.
Guaranteed A Grade: All students who use our services pass with 90%
guarantee.
Related Questions
Correct Answer is B
Explanation
The hypothesis should be modified to include the new findings that worm infestation can relieve the effects of certain autoimmune disorders.
A possible modification could be: “Parasitic worm infestation can have both damaging and beneficial effects on the host.
While it can cause harm, it has also been found to reduce the severity of certain autoimmune disorders.”
Choice A.
Worm infestation prevents the body from immune malfunction is not correct because it overstates the findings and implies that worm infestation completely prevents immune malfunction, which is not supported by the evidence.
Choice C.
Worm infestations exacerbate the body’s immune reactions is not correct because it contradicts the new findings that worm infestation can relieve the effects of certain autoimmune disorders.
Choice D.
Lack of worm infestations is the cause of some autoimmune disorders is not correct because it overstates the findings and implies a causal relationship between lack of worm infestations and autoimmune disorders, which is not supported by the evidence.
Correct Answer is D
Explanation
Sodium bicarbonate neutralizes the acidity of chyme.
The pancreas secretes large amounts of sodium bicarbonate, which protects the duodenum by neutralizing the acid that comes from the stomach.
This compound helps neutralize stomach acid generated during the digestive process.
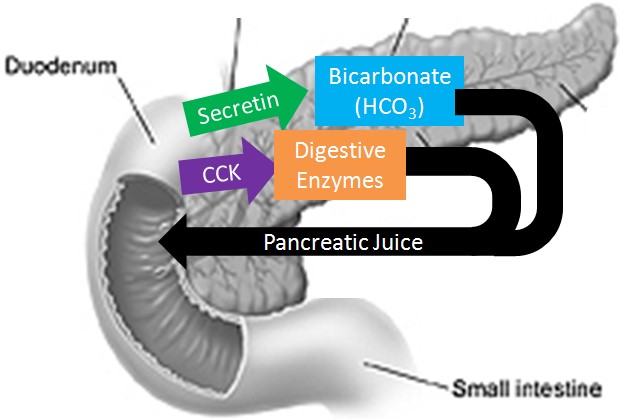
Choice A is incorrect because sodium bicarbonate is not a protease that digests carbohydrates.
Proteases are enzymes that break down proteins, while sodium bicarbonate is a chemical compound that helps neutralize stomach acid.
Choice B is incorrect because sodium bicarbonate does not stimulate the pyloric sphincter.
The pyloric sphincter is a ring of smooth muscle that separates the stomach from the duodenum and regulates the passage of partially digested food (chyme) into the small intestine.
Choice C is incorrect because sodium bicarbonate does not inhibit peristalsis.
Peristalsis is a series of wave-like muscle contractions that move food through the digestive tract.
Correct Answer is C
Explanation
The two major parts of the nervous system are the Central Nervous System (CNS) and the Peripheral Nervous System (PNS).
The CNS is made up of the brain and spinal cord and acts as the integration and command center of the body.
The PNS represents the conduit between the CNS and the body and is further subdivided into the somatic nervous system (SNS) and the autonomic nervous system (ANS).
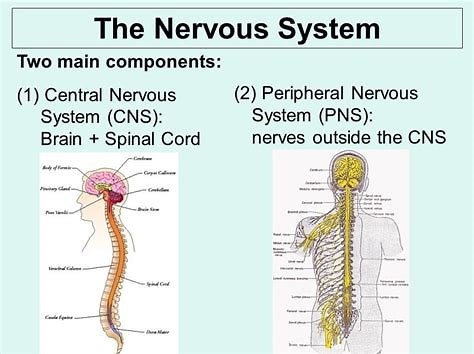
Choice A is incorrect because it only mentions two subdivisions of the PNS, which are the autonomic nervous system (ANS) and somatic nervous system (SNS).
Choice B is incorrect because it only mentions one major part of the nervous system, which is the PNS, and one subdivision of it, which is the SNS.
Choice D is incorrect because it only mentions one major part of the nervous system, which is the CNS, and one subdivision of the PNS, which is the ANS.
Correct Answer is C
Explanation
The best reason for the prolonged preservation of the body is that it was frozen in the cold temperature of the Alps shortly after he died and remained frozen until it was found.
Freezing can preserve a body by slowing down or stopping the decomposition process.
Choice A is not correct because the food that the person ate would not have contained toxins that killed the bacteria that would have otherwise destroyed the body.
Choice B is not correct because the arrow wound would not have caused blood to flow out of the body in a way that would have cleared enzymes that break down tissue from the body.
Choice D is not correct because ultraviolet rays at high altitude would not have caused all of the body’s molecules to be preserved.
Correct Answer is D
Explanation
Viruses.
Viruses lack essential machinery needed to reproduce by themselves.
In fact, viruses can only reproduce after infecting a living cell - a process called viral replication.
Once inside a living cell, viruses re-program the cell’s machinery to produce viral proteins and genetic material to make new copies of themselves.
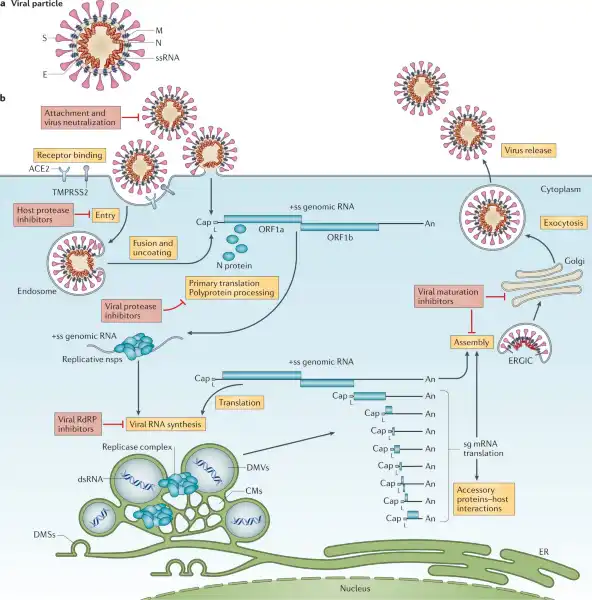
Choice A, Bacteria, is not the correct answer because bacteria have their own metabolic pathways and can reproduce outside of a host cell.
Choice B, Protozoa, is also not the correct answer because protozoa are singlecelled eukaryotes that have their own metabolic pathways and can reproduce outside of a host cell.
Choice C, Helminths, is not the correct answer because helminths are multicellular parasitic worms that have their own metabolic pathways and can reproduce outside of a host cell.
Correct Answer is D
Explanation
The cytoskeleton of a cell is comprised of protein fibers that provide structural support and help maintain the shape of the cell.
These protein fibers include microfilaments, intermediate filaments, and microtubules.
Choice A.
Carbohydrates is not the correct answer because carbohydrates are a type of macromolecule that provides energy to cells and are not a component of the cytoskeleton.
Choice B.
Nucleic acids is not the correct answer because nucleic acids are macromolecules that store and transmit genetic information and are not a component of the cytoskeleton.
Choice C.
Lipids is not the correct answer because lipids are a type of macromolecule that makes up cell membranes and are not a component of the cytoskeleton.
Correct Answer is B
Explanation
Antidiuretic hormone (ADH), also known as vasopressin, is a hormone that helps regulate the amount of water in your body.
It works to control the amount of water your kidneys reabsorb as they filter out waste from your blood.
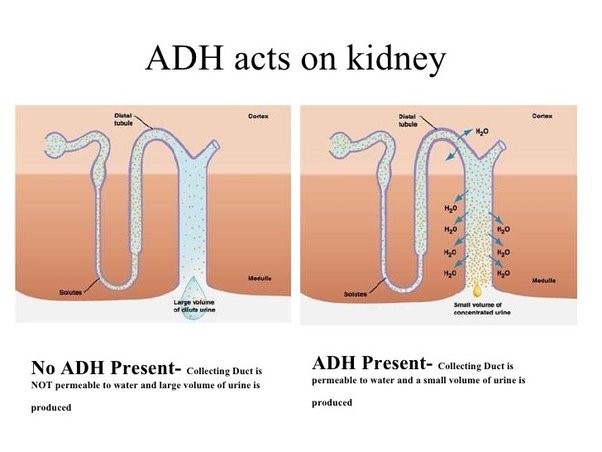
Choice A is not correct because an increase in the concentration of calcium in the glomerulus is not a physiological response caused by the release of antidiuretic hormone.
Choice C is not correct because a decrease in the concentration of calcium in the glomerulus is not a physiological response caused by the release of antidiuretic hormone.
Choice D is not correct because a decrease in water reabsorption in the collecting duct is not a physiological response caused by the release of antidiuretic hormone.
Correct Answer is D
Explanation
Genes that regulate cell division are found in some viruses.
When viruses cause an infection, they spread their DNA, affecting healthy cells’ genetic makeup and potentially causing them to turn into cancer.
For instance, HPV infections cause the virus’ DNA to combine with the host’s DNA, disrupting the normal function of cells.
Choice A is not correct because cancerous and normal cells sharing genetic sequences does not support the hypothesis that viruses can cause cancer.
Choice B is not correct because cellular DNA having sequences related to viral sequences does not support the hypothesis that viruses can cause cancer.
Choice C is not correct because viruses and cancer cells both replicating rapidly does not support the hypothesis that viruses can cause cancer.
Correct Answer is B
Explanation
Electrophoresis is the most useful laboratory method for separating genomic DNA fragments by size.
Electrophoresis is a technique that uses an electric field to separate charged molecules, such as DNA fragments, based on their size and charge.
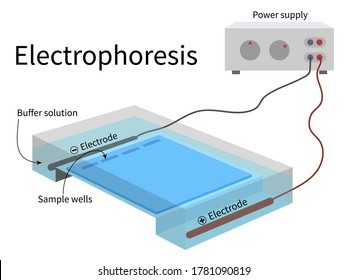
Choice A is not correct because titration is a laboratory method used to determine the concentration of a solution.
Choice C is not correct because filtration is a laboratory method used to separate solids from liquids.
Choice D is not correct because spectrophotometry is a laboratory method used to measure the absorbance of light by a solution.
Correct Answer is B
Explanation
The atomic number of an element represents the number of protons in the nucleus of an atom of that element.
Since lithium has an atomic number of 3, it has 3 protons in its nucleus.

Choice A is not correct because 7 is the mass number of lithium, not the number of protons.
Choice C is not correct because 12 is not the atomic number or mass number of lithium.
Choice D is not correct because 4 is not the atomic number or mass number of lithium.
This question was extracted from the actual TEAS Exam. Ace your TEAS exam with the actual TEAS 7 questions, Start your journey with us today
Visit Naxlex, the Most Trusted TEAS TEST Platform With Guaranteed Pass of 90%.
Money back guarantee if you use our service and fail the actual exam. Option of personalised live tutor on your area of weakness.
