Which of the following substances is responsible for donating H+ ions to act as a buffer when blood pH rises?
Carbon dioxide
Carbon monoxide
Carbonic acid
Oxygen
Correct Answer : C
Carbonic acid.
In the human body, maintaining the pH of the blood within a narrow range is critical for proper physiological functioning.
One of the buffering systems that helps to regulate blood pH involves the conversion of carbon dioxide (CO2) and water (H2O) into carbonic acid (H2CO3), which then dissociates into hydrogen ions (H+) and bicarbonate ions (HCO3-).
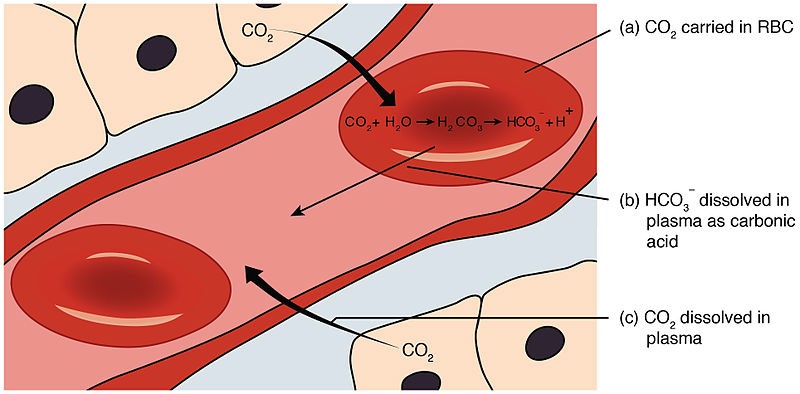
Carbonic acid (H2CO3) is responsible for donating H+ ions to act as a buffer when blood pH rises.
When blood pH rises (becomes more alkaline), carbonic acid dissociates, and the H+ ions combine with bicarbonate ions to form more carbonic acid.
This helps to remove excess H+ ions from the blood and prevent the pH from rising too much.
Option A, carbon dioxide, is involved in the buffering system through its conversion to carbonic acid.
However, it does not directly donate H+ ions to act as a buffer when blood pH rises.
Option B, carbon monoxide, is a toxic gas that binds to hemoglobin in red blood cells, preventing them from carrying oxygen.
It is not involved in the buffering system and does not donate H+ ions.
Option D, oxygen, is carried by hemoglobin in red blood cells and is essential for respiration.
It is not involved in the buffering system and does not donate H+ ions.
TEAS 7 Exam Quiz Bank
HESI A2 Exam Quiz Bank
Find More Questions 📚
Teas 7 Questions: We got the latest updated TEAS 7 questions
100% Money Refund: 100% money back guarantee if you take our full
assessment pass with 80% and fail the actual exam.
Live Tutoring: Fully customized live tutoring lessons.
Guaranteed A Grade: All students who use our services pass with 90%
guarantee.
Related Questions
Correct Answer is D
Explanation
Microtubule organization.
Centrosomes are organelles that serve as the main microtubule-organizing centers for animal cells.
They regulate the movement of microtubules and other cytoskeletal structures, thereby facilitating changes in the shapes of the membranes of animal cells.
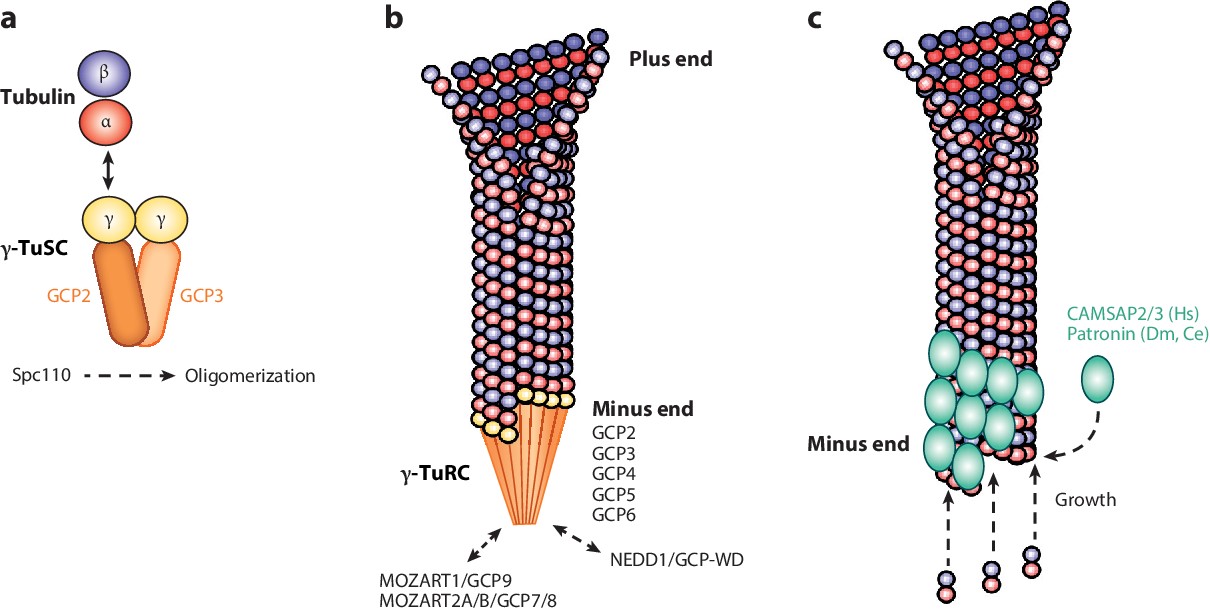
Choice A, Organelle trafficking, is not the correct answer because while centrosomes do play a role in intracellular trafficking during interphase by organizing an astral ray of microtubules, their main function is microtubule organization.
Choice B, Pathogen digestion, is not the correct answer because centrosomes do not play a direct role in pathogen digestion.
Choice C, Cytoplasm formation, is not the correct answer because centrosomes do not play a direct role in cytoplasm formation.
Correct Answer is C
Explanation
Osmosis is the movement of water molecules across a selectively permeable membrane from an area of higher water concentration to an area of lower water concentration.
In a hypertonic solution, the concentration of solutes outside the cell is higher than inside the cell, so water flows out of the cell through aquaporins embedded in the plasma membrane to balance the concentration gradient.
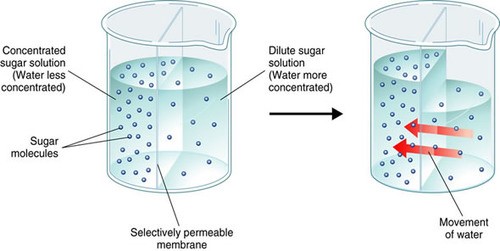
Choice A.
Facilitated diffusion is not correct because it is a type of passive transport that involves the movement of molecules across a membrane through specific transport proteins, but it does not specifically refer to the movement of water molecules.
Choice B.
Active transport is not correct because it is a type of transport that involves the movement of molecules against their concentration gradient and requires energy in the form of ATP, but osmosis is a passive process that does not require energy.
Choice D.
Diffusion is not correct because it refers to the movement of molecules from an area of higher concentration to an area of lower concentration, but it does not specifically refer to the movement of water molecules.
Correct Answer is D
Explanation
Proteins.
Proteins are made up of amino acids which are organic molecules that contain both an amine functional group (–NH2) and a carboxylic acid functional group (– COOH).
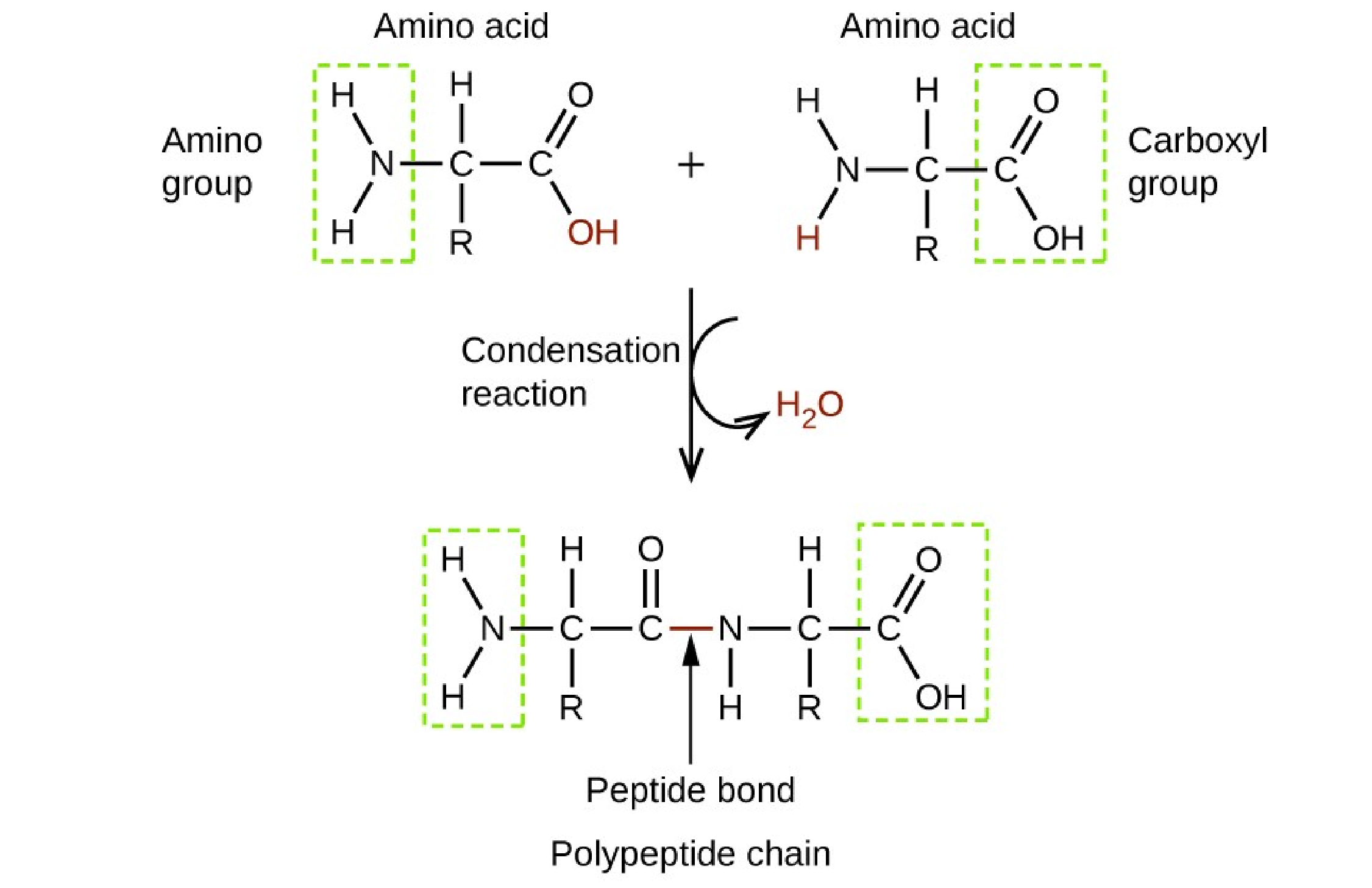 |
Choice A, Lipids, is not the correct answer because lipids are a group of naturally occurring molecules that include fats, waxes, sterols, fat-soluble vitamins, monoglycerides, diglycerides, triglycerides, phospholipids, and others.
They do not contain both an amine and carboxyl group.
Choice B, Chitin, is not the correct answer because chitin is a long-chain polymer of N-acetylglucosamine, a derivative of glucose.
It does not contain both an amine and carboxyl group.
Choice C, Cellulose, is not the correct answer because cellulose is an organic compound with the formula (C6H10O5)n, a polysaccharide consisting of a linear chain of several hundred to many thousands of β(1→4) linked D-glucose units.
It does not contain both an amine and carboxyl group.
Correct Answer is C
Explanation
Carbonic acid.
In the human body, maintaining the pH of the blood within a narrow range is critical for proper physiological functioning.
One of the buffering systems that helps to regulate blood pH involves the conversion of carbon dioxide (CO2) and water (H2O) into carbonic acid (H2CO3), which then dissociates into hydrogen ions (H+) and bicarbonate ions (HCO3-).

Carbonic acid (H2CO3) is responsible for donating H+ ions to act as a buffer when blood pH rises.
When blood pH rises (becomes more alkaline), carbonic acid dissociates, and the H+ ions combine with bicarbonate ions to form more carbonic acid.
This helps to remove excess H+ ions from the blood and prevent the pH from rising too much.
Option A, carbon dioxide, is involved in the buffering system through its conversion to carbonic acid.
However, it does not directly donate H+ ions to act as a buffer when blood pH rises.
Option B, carbon monoxide, is a toxic gas that binds to hemoglobin in red blood cells, preventing them from carrying oxygen.
It is not involved in the buffering system and does not donate H+ ions.
Option D, oxygen, is carried by hemoglobin in red blood cells and is essential for respiration.
It is not involved in the buffering system and does not donate H+ ions.
Correct Answer is C
Explanation
Ovulation is the process in which an ovarian follicle matures and releases a reproductive egg.
During ovulation, a mature egg is released from the female ovary, enabling it to be fertilized by male sperm cells 1.
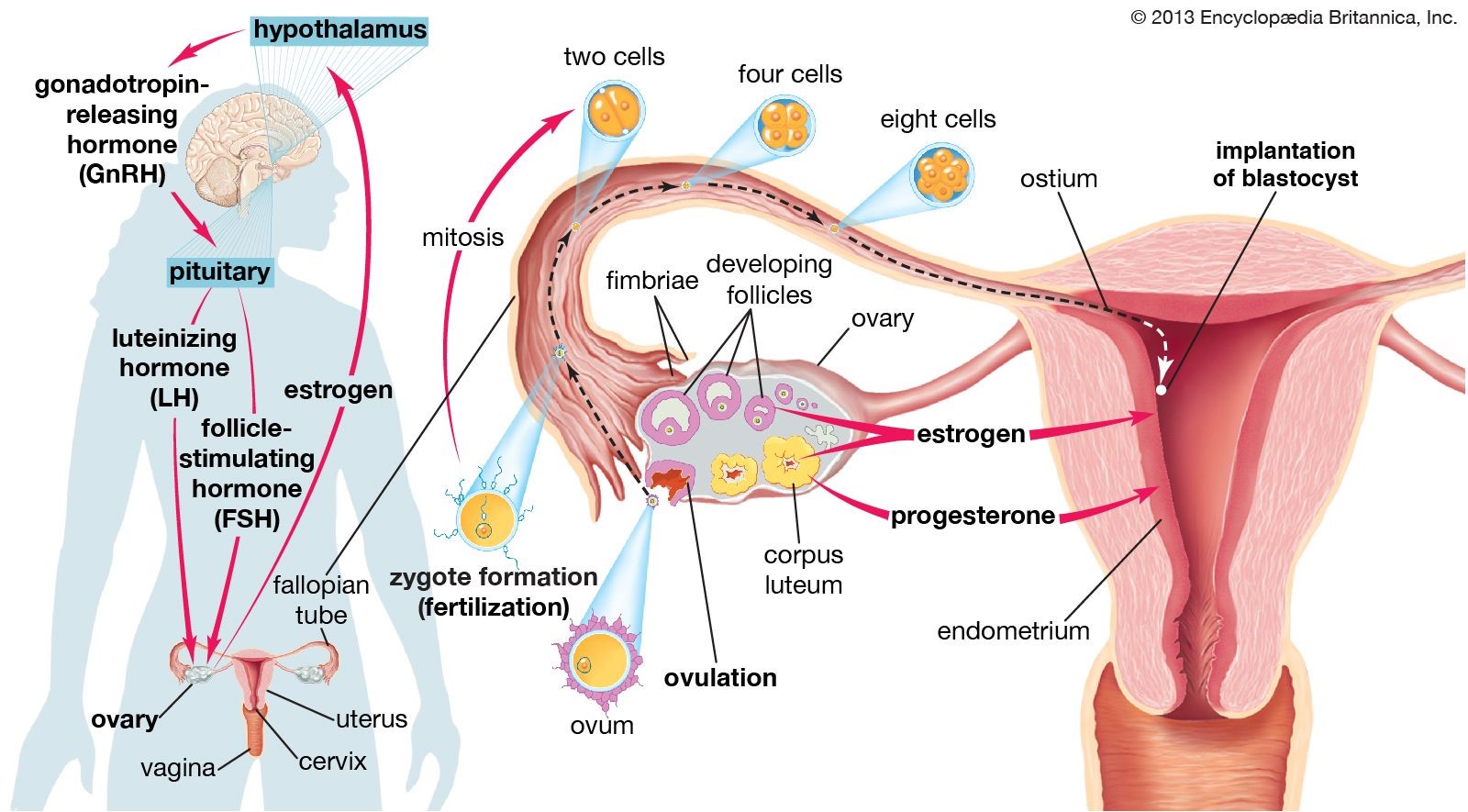
Choice A is incorrect because menstruation is the process of shedding the uterine lining, which occurs when an egg is not fertilized.
Choice B is incorrect because fertilization is the process of a sperm cell joining with an egg cell to form a zygote.
Choice D is incorrect because oogenesis is the process of forming female gametes (eggs) in the ovaries.
Correct Answer is B
Explanation
The corpus luteum is a structure that develops in the ovary after an egg has been released.
It secretes the hormone progesterone, which prepares the uterus for a fertilized egg to implant and helps maintain the uterine lining during pregnancy1.
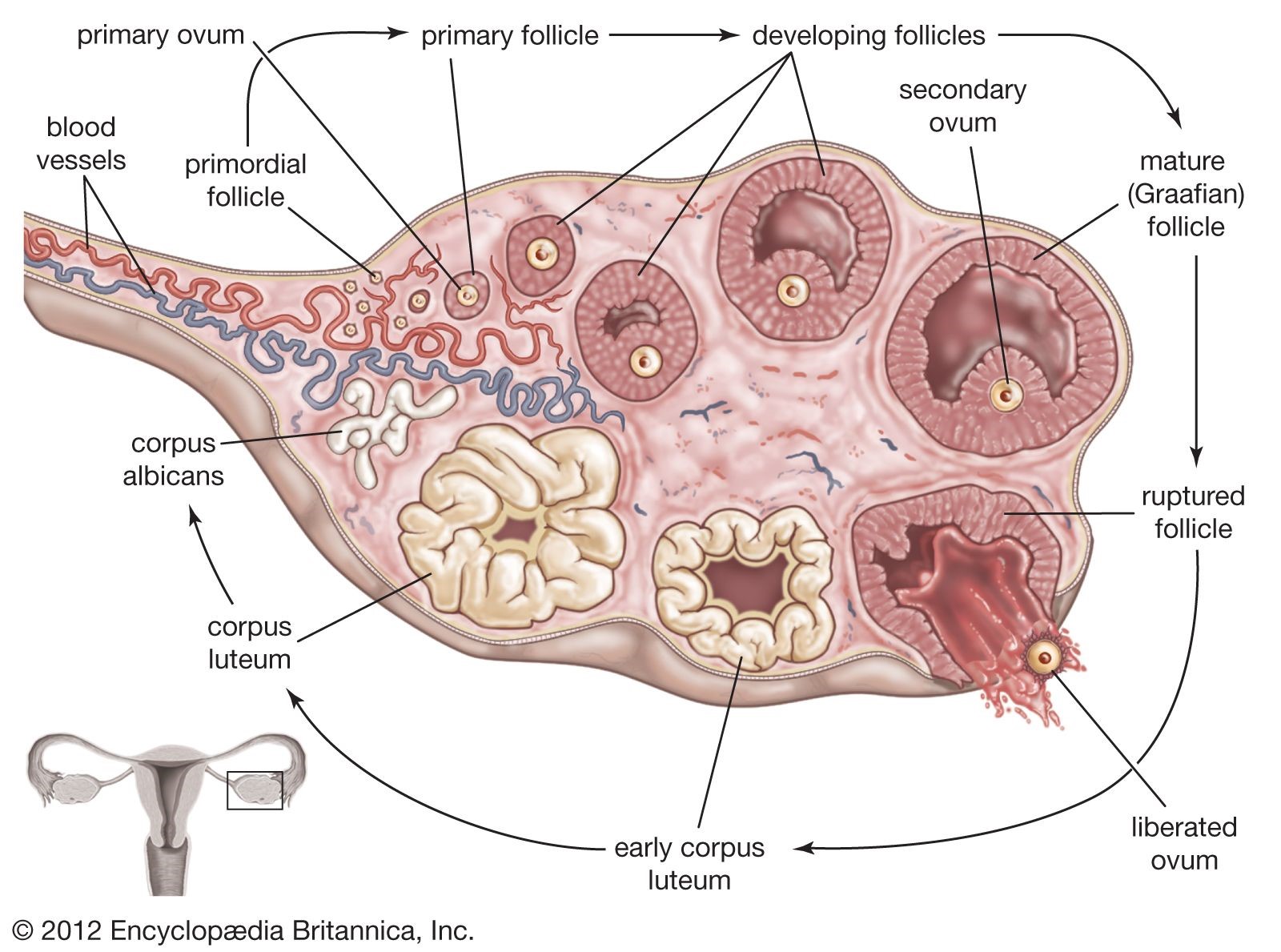
Choice A.
Umbilical cord is not correct because it is a structure that connects the developing fetus to the placenta and provides nutrients and oxygen to the fetus, but does not secrete hormones.
Choice C.
Oviduct is not correct because it is a tube that transports eggs from the ovary to the uterus, but does not secrete hormones.
Choice D.
Oocyte is not correct because it is an immature egg cell, but does not secrete hormones.
Correct Answer is A
Explanation
The approximate threshold value for mammalian neurons is -55 mV.
The threshold potential is the critical level to which a membrane potential must be depolarized to initiate an action potential.
Most often, the threshold potential is a membrane potential value between –50 and –55 mV
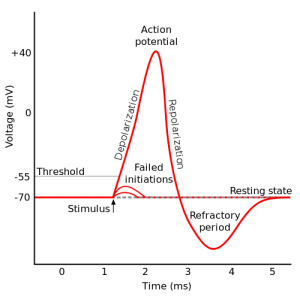
The membrane potential of a neuron is determined by the distribution of ions across the cell membrane.
At rest, the inside of a neuron is more negative than the outside due to the presence of negatively charged proteins and other molecules.
The movement of ions across the cell membrane can change the membrane potential.
For example, when sodium ions enter the cell, they make the inside of the cell more positive (less negative), causing depolarization.
Choice B is incorrect because -80 mV is below the typical threshold value for mammalian neurons.
Choice C is incorrect because +35 mV is above the typical threshold value for mammalian neurons.
Choice D is incorrect because 0 mV is above the typical threshold value for mammalian neurons.
Correct Answer is B
Explanation
Electrophoresis is the most useful laboratory method for separating genomic DNA fragments by size.
Electrophoresis is a technique that uses an electric field to separate charged molecules, such as DNA fragments, based on their size and charge.
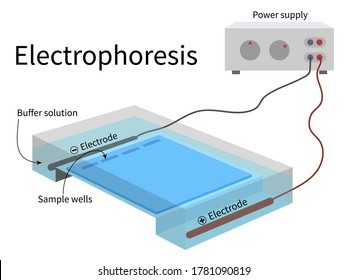
Choice A is not correct because titration is a laboratory method used to determine the concentration of a solution.
Choice C is not correct because filtration is a laboratory method used to separate solids from liquids.
Choice D is not correct because spectrophotometry is a laboratory method used to measure the absorbance of light by a solution.
Correct Answer is A
Explanation
Triple point.
In a phase diagram, the term used for a substance held at a temperature and pressure where the solid, liquid, and gaseous states of a substance exist simultaneously is the triple point.
The triple point is a unique point on a phase diagram where the three states of matter (solid, liquid, and gas) can coexist in equilibrium.
At the triple point, the temperature and pressure of the substance are fixed.
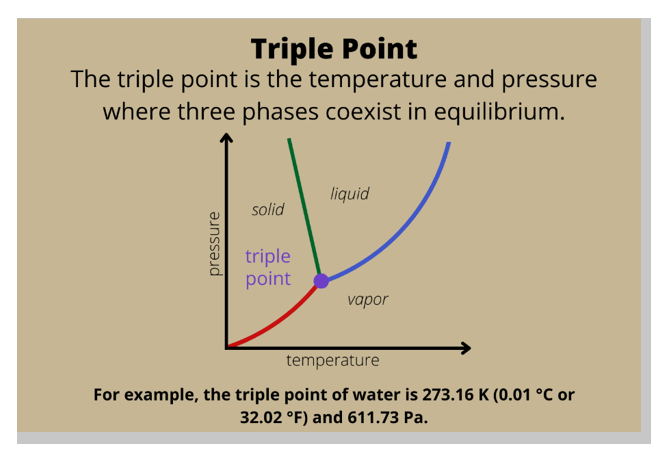
Option B, critical temperature, is the temperature at which a gas cannot be liquefied, regardless of the pressure applied.
It is a characteristic property of a substance and is typically higher than the boiling point of the liquid at standard pressure.
Option C, critical point, is the point on a phase diagram where the liquid and gas phases of a substance become indistinguishable.
At the critical point, the distinction between the liquid and gas phases disappears, and the substance becomes a supercritical fluid.
Option D, absolute zero, is the theoretical temperature at which all matter has zero thermal energy.
At absolute zero, all substances are in their solid state, but it is not relevant to a phase diagram, as it is a temperature where no transitions between states occur.
In summary, the term used for a substance held at a temperature and pressure where the solid, liquid, and gaseous states of a substance exist simultaneously in a phase diagram is the triple point, whereas the other options provided are not relevant or are characteristic properties of substances in different contexts.
Correct Answer is D
Explanation
The pleura is a double-layered serous membrane that covers each lung and lines the thoracic cage
The pleura is a vital part of the respiratory tract.
Its role is to cushion the lung and reduce any friction that may develop between the lung, rib cage, and chest cavity.
Each pleura (there are two) consists of a two-layered membrane that covers each lung.
The layers are separated by a small amount of viscous (thick) lubricant known as pleural fluid.
The pleura is comprised of two distinct layers: the visceral pleura and the parietal pleura.
The visceral pleura is the thin, slippery membrane that covers the surface of the lungs and dips into the areas separating the different lobes of the lungs (called the hilum).
This question was extracted from the actual TEAS Exam. Ace your TEAS exam with the actual TEAS 7 questions, Start your journey with us today
Visit Naxlex, the Most Trusted TEAS TEST Platform With Guaranteed Pass of 90%.
Money back guarantee if you use our service and fail the actual exam. Option of personalised live tutor on your area of weakness.
