A client is diagnosed with syndrome of inappropriate antidiuretic hormone (SIADH).
The nurse would monitor the client for which of the following electrolyte imbalances?
Hyponatremia.
Hypernatremia.
Hyperkalemia.
Hypokalemia.
The Correct Answer is A
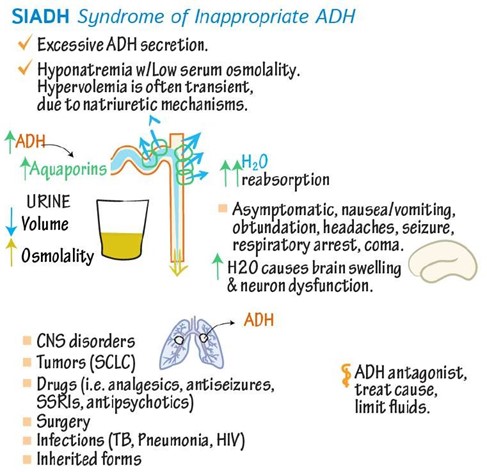
SIADH is a condition in which your body makes too much antidiuretic hormone (ADH), which controls how your body releases and conserves water.
SIADH makes it harder for your kidneys to release water and causes the levels of electrolytes, like sodium, to fall due to water retention.
This leads to hyponatremia, which is when you have low levels of sodium in your blood.
Choice B is wrong because hypernatremia is when you have high levels of sodium in your blood.
This can occur due to dehydration, excessive salt intake, or kidney problems.
Choice C is wrong because hyperkalemia is when you have high levels of potassium in your blood.
This can occur due to kidney failure, acidosis, or certain medications.
Choice D is wrong because hypokalemia is when you have low levels of potassium in your blood.
This can occur due to vomiting, diarrhea, diuretics, or alkalosis.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
Correct Answer is A
Explanation

Oral potassium supplements can cause stomach irritation or laxative effect if taken without enough fluid or food.
Taking the medication with food or a full glass of water can help prevent these side effects and improve absorption.
Choice B is wrong because taking the medication on an empty stomach with a sip of water can increase the risk of stomach irritation or laxative effect and reduce absorption.
Choice C is wrong because taking the medication with milk or antacids can interfere with the absorption of potassium and cause hyperkalemia (high blood potassium levels).
Choice D is wrong because taking the medication with grapefruit juice can also interfere with the absorption of potassium and cause hyperkalemia.
Grapefruit juice can also interact with some medications that affect potassium levels, such as angiotensin-converting enzyme (ACE) inhibitors and potassium- sparing diuretics.
Correct Answer is C
Explanation
This is because acute renal failure is a condition where the kidneys lose their ability to filter waste and excess fluid from the blood. This can lead to fluid overload, electrolyte imbalances, and metabolic acidosis. Therefore, the nurse should monitor the patient’s urine output and fluid balance to assess the severity of the renal impairment and prevent complications.
Choice A is wrong because administering a potassium-sparing diuretic would worsen the patient’s hyperkalemia, which is a common complication of acute renal failure.
Choice B is wrong because encouraging the patient to consume a high-sodium diet would increase the patient’s fluid retention and blood pressure, which can further damage the kidneys.
Choice D is wrong because administering intravenous antibiotics is not a priority intervention for acute renal failure unless there is a specific indication of infection.
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
