A patient with chronic kidney disease is at risk for developing which of the following electrolyte imbalances?
Decrease in the concentration of calcium in the glomerulus.
Increase in the concentration of potassium in the blood.
Decrease in the concentration of sodium in the blood.
Increase in the concentration of magnesium in the blood.
The Correct Answer is B
A patient with chronic kidney disease is at risk for developing an increase in the concentration of potassium in the blood.
The kidneys play a pivotal role in the regulation of electrolyte balance.
With the progressive loss of kidney function, derangements in electrolytes inevitably occur and contribute to poor patient outcomes123.
Choice A is incorrect because calcium concentration is not regulated in the glomerulus.
Choice C is incorrect because chronic kidney disease can result in either an increase or decrease in sodium concentration in the blood.
Choice D is incorrect because chronic kidney disease does not necessarily result in an increase in magnesium concentration in the blood.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
Correct Answer is C
Explanation
Inflammatory cytokines released during the early response to bacterial infection play a crucial role in initiating cell recruitment and local inflammation 1.
They induce the expression of adhesion molecules in endothelial cells and promote the recruitment of neutrophils to the site of inflammation 1.
Choice A is incorrect because while inflammatory cytokines may enhance phagocytosis, they do not directly disrupt the infection.
Choice B is incorrect because inflammatory cytokines do not directly attack invading pathogens.
Choice D is incorrect because inflammatory cytokines do not secrete antibodies to neutralize pathogens.
Correct Answer is D
Explanation
Atomic mass is very close to mass number but with some deviation in the decimal places.
Atomic mass is also known as atomic weight and is the weighted average mass of an atom of an element based on the relative natural abundance of that element’s isotopes.
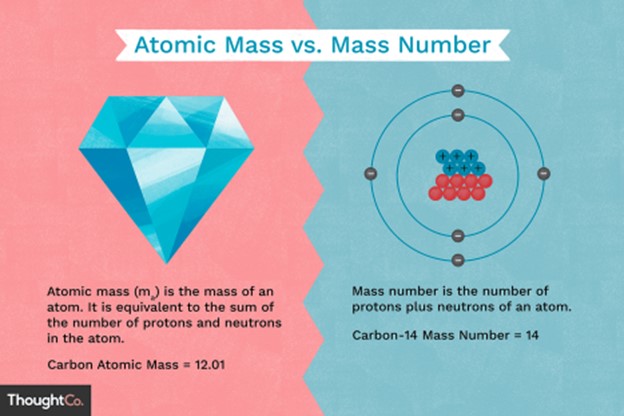
The mass number, on the other hand, is a count of the total number of protons and neutrons in an atom’s nucleus.
Choice A is incorrect because atomic mass and mass number do not mean the same thing.
Choice B is incorrect because atomic mass is not always greater than mass number.
Choice C is incorrect because atomic mass and mass number are related.
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
