What is the molecular geometry of a molecule of Sulphur dioxide (SO2)?
Linear
Trigonal planar
Bent
Tetrahedral
The Correct Answer is C
The molecular geometry of a molecule of Sulphur dioxide (SO2) is bent or V-shaped. This is because of the presence of two lone pairs on the sulfur atom, which cause repulsion and distort the bond angles in the molecule.
SO2 has a central sulfur atom bonded to two oxygen atoms by double bonds. The two double bonds and the two lone pairs of electrons on the sulfur result in a trigonal planar arrangement of electron pairs around the sulfur atom. However, the repulsion between the lone pairs causes the two oxygen atoms to be pulled closer together, resulting in a bent or V-shaped molecular geometry.
The bent molecular geometry of SO2 affects its properties, such as its polarity and reactivity. SO2 is a polar molecule due to the asymmetric distribution of electrons, which results in a partial positive charge on the sulfur atom and partial negative charges on the oxygen atoms. This polarity makes SO2 a good solvent and reactant in chemical reactions, as well as a contributor to air pollution and acid rain.

Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
Correct Answer is A
Explanation
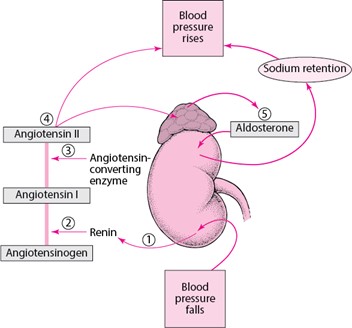
Renin is an enzyme that is produced by the kidneys and it acts to elevate blood pressure. When blood pressure falls, the kidneys secrete renin into the bloodstream
Correct Answer is B
Explanation
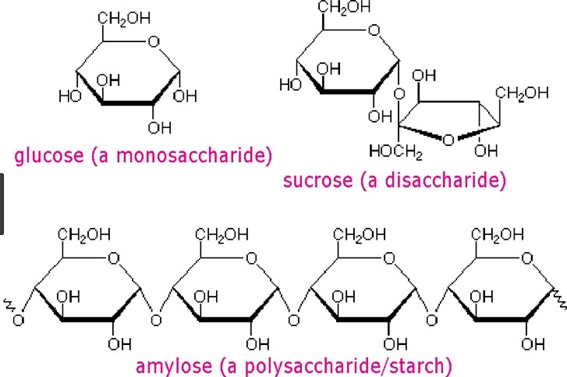
Monosaccharides are simple sugars that cannot be further broken down into simpler sugars while disaccharides are composed of two simple sugars.
Carbohydrates are one of the main types of biomolecules and are composed of monomers called monosaccharides. Monosaccharides are simple sugars that cannot be further broken down into simpler sugars. They are usually composed of 3 to 7 carbon atoms and have a general formula of (CH2O)n, where n is a number between 3 and 7. Examples of monosaccharides include glucose, fructose, and galactose.
When two monosaccharides are joined together by a glycosidic bond, they form a disaccharide. Disaccharides are composed of two simple sugars and can be broken down into their constituent monosaccharides by hydrolysis. Examples of disaccharides include sucrose, lactose, and maltose.
Option a) is incorrect because it describes the composition of a disaccharide, not a monosaccharide.
Option c) is incorrect because both monosaccharides and disaccharides can be found in both plants and animals.
Option d) is incorrect because both monosaccharides and disaccharides can be used for energy storage and
structural purposes, depending on their specific structure and function in the organism.
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
