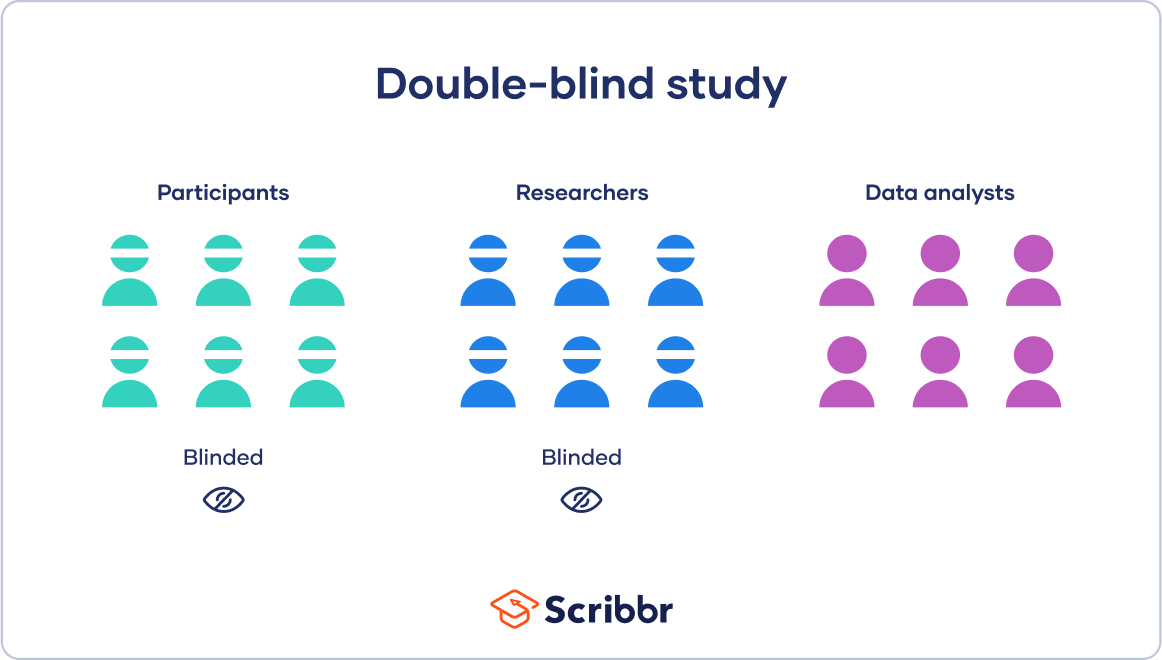
Which of the following is an example of a double-blind study?
Participants are randomly assigned to a treatment group or a control group
Participants and researchers both know which group participants are assigned to
Participants do not know which group they are assigned to, but researchers do
Both participants and researchers do not know which group participants are assigned to
Correct Answer : D
A double-blind study is a research design in which neither the participants nor the researchers know which group participants are assigned to. This is done to minimize bias and ensure that the results of the study are as objective as possible. In a double-blind study, the treatment and control groups are randomly assigned, and the participants and researchers are unaware of which group each participant is assigned to. Option a) is an example of a randomized controlled trial, which is a common research design, but it is not necessarily double-blind. Option b) is an example of an open-label study, in which both the participants and the researchers know which group each participant is assigned to. Option c) is an example of a single-blind study, in which the participants do not know which group they are assigned to, but the researchers do.
TEAS 7 Exam Quiz Bank
HESI A2 Exam Quiz Bank
Find More Questions 📚
Teas 7 Questions: We got the latest updated TEAS 7 questions
100% Money Refund: 100% money back guarantee if you take our full
assessment pass with 80% and fail the actual exam.
Live Tutoring: Fully customized live tutoring lessons.
Guaranteed A Grade: All students who use our services pass with 90%
guarantee.
Related Questions
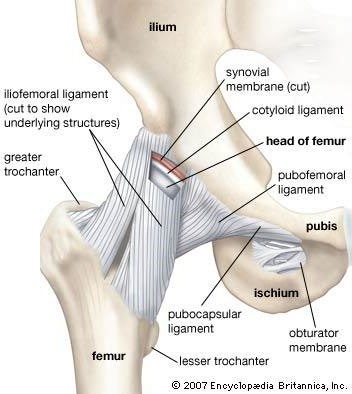
Correct Answer is B
Explanation
Ligaments are tough bands of fibrous tissue that connect two bones together in a joint. They provide stability and support to the joint, preventing excessive movement and helping to maintain proper alignment of the bones.
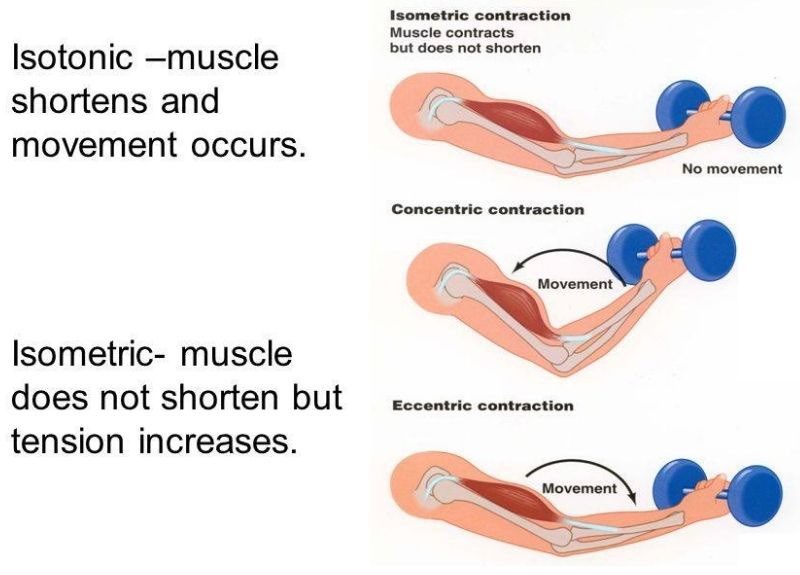
Correct Answer is B
Explanation
Isotonic and isometric contractions are two types of muscle contractions that differ in the amount of force produced and the movement of the muscle. In isotonic contractions, the muscle changes length and produces movement, such as lifting a weight. The force generated by the muscle remains constant throughout the movement. Isotonic contractions can be further classified as concentric contractions, in which the muscle shortens as it contracts, and eccentric contractions, in which the muscle lengthens as it contracts.
In contrast, isometric contractions occur when the muscle generates force without changing its length or producing movement. For example, holding a weight in a fixed position without moving it requires an isometric contraction. In an isometric contraction, the force generated by the muscle increases up to a maximum and then remains constant. Isometric contractions can be used to build strength and endurance in the muscle, but they do not produce movement.
 |
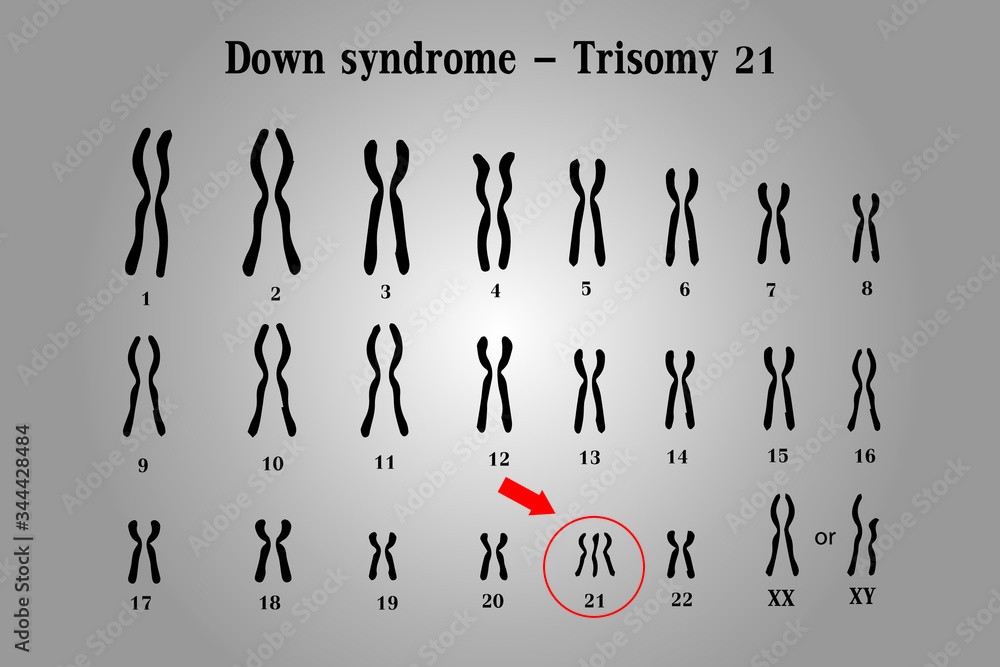
Correct Answer is C
Explanation
Down syndrome is a genetic disorder caused by the presence of an extra copy of chromosome 21. It is also known as trisomy 21, because affected individuals have three copies of chromosome 21 instead of the normal two.
The extra chromosome 21 in Down syndrome occurs due to a random error in cell division, which leads to the production of an abnormal gamete (egg or sperm) with an extra copy of the chromosome. When this gamete fuses with a normal gamete during fertilization, the resulting zygote has 47 chromosomes instead of the usual 46, and develops into a fetus with Down syndrome.
Down syndrome is characterized by a range of physical and intellectual symptoms, including developmental delays, intellectual disability, distinctive facial features, heart defects, and increased risk of certain medical conditions such as leukemia and Alzheimer's disease. However, the severity and expression of these symptoms can vary widely among affected individuals.
 |
Correct Answer is D
Explanation
Exocrine glandular is not one of the four primary tissue types found in the human body. The four primary tissue types are epithelial, nervous, connective, and muscle.
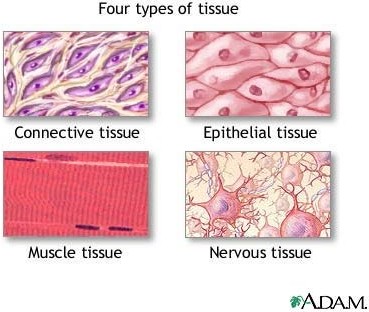 |
Correct Answer is D
Explanation
The unit used to indicate length is the meter (m). It is the base unit of length in the International System of Units (SI).
Correct Answer is B
Explanation
The mitral valve is located between the left atrium and left ventricle of the heart and helps to regulate the flow of blood between these chambers. It consists of two leaflets or flaps that open and close in response to changes in pressure as the heart beats.
During diastole, when the heart is relaxed and filling with blood, the mitral valve opens to allow blood to flow from the left atrium into the left ventricle. During systole, when the heart contracts to pump blood out of the left ventricle and into the systemic circulation, the mitral valve closes to prevent backflow of blood into the left atrium.
The mitral valve is one of four valves in the heart that help to ensure the unidirectional flow of blood through the heart and the rest of the circulatory system. Problems with the mitral valve, such as mitral valve prolapse or mitral stenosis, can lead to a range of symptoms and complications, including shortness of breath, fatigue, chest pain, and heart failure.
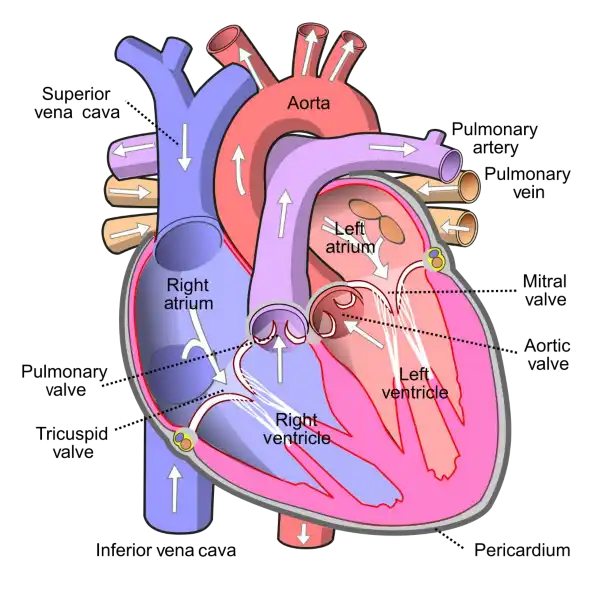 |
Correct Answer is A
Explanation
The diaphragm is a dome-shaped muscle that plays a key role in breathing. It separates the thoracic cavity, which contains the heart and lungs, from the abdominal cavity. When the diaphragm contracts, it moves downward and increases the volume of the thoracic cavity, allowing air to flow into the lungs. When it relaxes, it moves upward and decreases the volume of the thoracic cavity, forcing air out of the lungs.
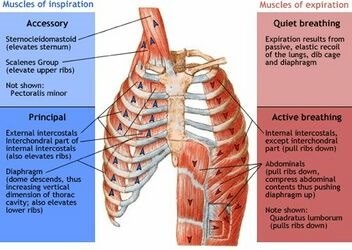 |
Correct Answer is B
Explanation
The epididymis is a coiled tube located at the back of each testicle where the sperm mature and are stored until ejaculation. Sperm are produced in the testes and then transported to the epididymis where they undergo maturation and become motile. The epididymis provides a protective environment for the sperm, allowing them to mature and become more resilient to external stressors. During ejaculation, the sperm are transported from the epididymis to the vas deferens and then to the urethra for ejaculation.
 |
Correct Answer is C
Explanation
The three major pairs of salivary glands are the parotid glands, sublingual glands, and submandibular glands.
- Parotid glands are located just in front of your ears.
- Sublingual glands are located below either side of your tongue, under the floor of your mouth.
- Submandibular glands are located below your jaw.
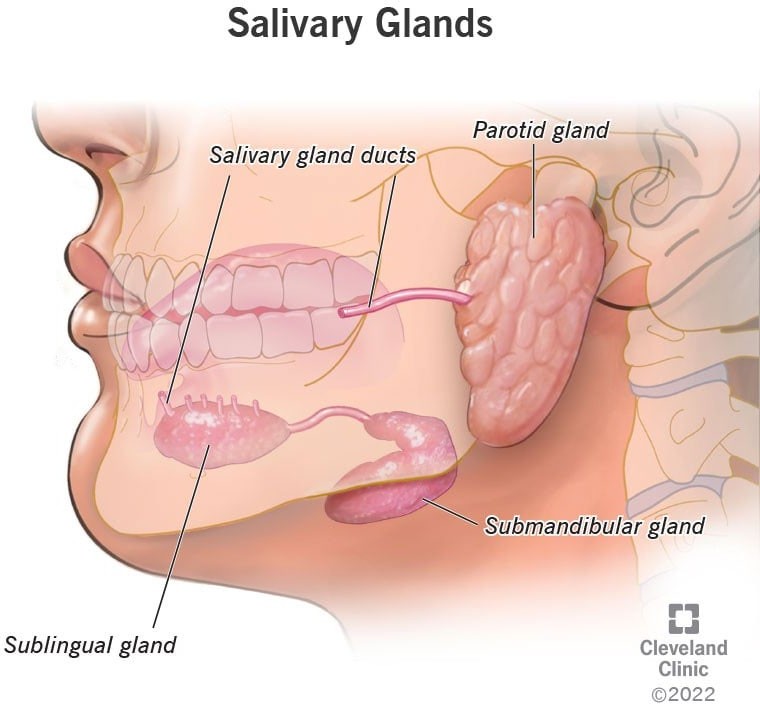 |
Correct Answer is C
Explanation
Vaccines are a type of preventative medicine that work by exposing the individual to a weakened or inactivated form of a pathogen (such as a virus or bacteria) or to a piece of the pathogen (such as a protein or sugar) that triggers an immune response in the body. This exposure allows the body to develop immunity to the pathogen without getting sick from the full-blown disease. Once the immune system has been primed, it can recognize and quickly respond to the pathogen if it is encountered again in the future, providing protection against the disease.
It is a common misconception that vaccines can cause the disease they are designed to protect against. This is not true. While some vaccines may cause mild symptoms such as a low-grade fever or soreness at the injection site, they do not cause the full-blown disease.
Vaccines provide active immunity, meaning that the body produces its own antibodies against the pathogen, rather than receiving pre-made antibodies as in passive immunity. Additionally, vaccines can be effective against both bacterial and viral infections, depending on the specific vaccine.
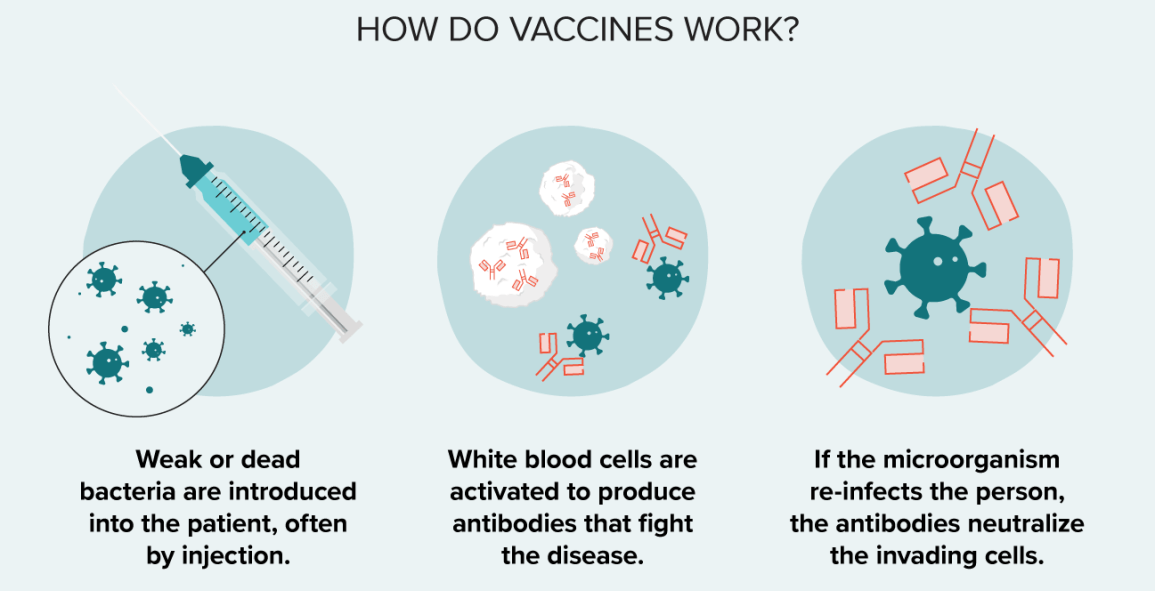
This question was extracted from the actual TEAS Exam. Ace your TEAS exam with the actual TEAS 7 questions, Start your journey with us today
Visit Naxlex, the Most Trusted TEAS TEST Platform With Guaranteed Pass of 90%.
Money back guarantee if you use our service and fail the actual exam. Option of personalised live tutor on your area of weakness.
