What is the difference between a physical change and a chemical change?
A physical change involves the rearrangement of atoms and molecules while a chemical change involves the formation of new substances with different chemical properties.
A physical change involves the change of one state of matter to another while a chemical change involves
the change of one substance into another.
A physical change involves the breaking of chemical bonds while a chemical change involves the breaking of intermolecular forces.
The Correct Answer is A
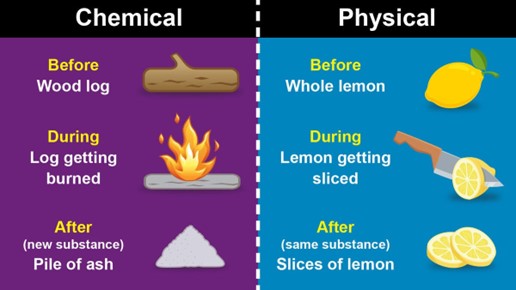
A physical change is a change that affects the physical properties of a substance but does not change its chemical identity. Physical changes include changes in state, such as melting or boiling, changes in shape or size, and changes in phase, such as the dissolution of a solid in a liquid. In a physical change, the atoms and molecules of the substance are rearranged, but no new substances are formed.
A chemical change, on the other hand, is a change that results in the formation of new substances with different chemical properties. Chemical changes involve the breaking of chemical bonds between atoms and the formation of new bonds to create new compounds. Chemical changes are usually accompanied by a change in color, the formation of a gas or a solid, or the release or absorption of energy.
Overall, the main difference between a physical change and a chemical change is that a physical change only affects the physical properties of a substance while a chemical change results in the formation of new substances with different chemical properties.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
Correct Answer is D
Explanation
The unit used to indicate length is the meter (m). It is the base unit of length in the International System of Units (SI).
Correct Answer is A
Explanation
Chlorophyll a is the primary pigment responsible for photosynthesis in plants. It is a green pigment that is essential for capturing light energy from the sun and converting it into chemical energy that can be used by the plant. Chlorophyll absorbs light most efficiently in the blue and red parts of the spectrum, and reflects green light, giving plants their characteristic green color
Chlorophyll b is another type of chlorophyll that is also involved in photosynthesis, but it is not as abundant as chlorophyll a. Chlorophyll b absorbs light most efficiently in the blue and orange parts of the spectrum and reflects yellow-green light.
Carotenoids are pigments that are present in many plants and are involved in photosynthesis as well as protecting the plant from damage caused by excess light. Carotenoids are responsible for the orange, yellow, and red colors of many fruits and vegetables.
Anthocyanins are pigments that give plants their red, purple, and blue colors. While they are not directly involved in photosynthesis, they play a role in attracting pollinators and protecting the plant from damage caused by UV radiation.
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
