Which of the following can be determined using culture and sensitivity tests?
Decision to administer either a bactericidal or bacteriostatic drug
Microbial susceptibility to an anti-infective
Duration of the antibacterial drug therapy
Decision to administer empiric therapy
The Correct Answer is B
A. Decision to administer either a bactericidal or bacteriostatic drug:
Culture and sensitivity tests provide information about the susceptibility of the microorganism to specific antimicrobial agents. Based on this information, healthcare providers can choose between bactericidal (agents that kill bacteria) or bacteriostatic (agents that inhibit bacterial growth) drugs. For example, if the culture indicates that the microorganism is susceptible to a bactericidal drug, such as penicillin, the healthcare provider may choose to administer that type of drug.
B. Microbial susceptibility to an anti-infective:
This option accurately describes one of the primary purposes of culture and sensitivity tests. These tests determine whether the microorganism causing the infection is susceptible or resistant to specific antimicrobial agents. This information guides the selection of the most appropriate anti-infective therapy to effectively treat the infection.
C. Duration of the antibacterial drug therapy:
While culture and sensitivity tests provide valuable information about microbial susceptibility to antimicrobial agents, they do not specifically determine the duration of antibacterial drug therapy. The duration of therapy is often determined based on factors such as the type and severity of the infection, the patient's response to treatment, and clinical guidelines, rather than solely on the results of culture and sensitivity tests.
D. Decision to administer empiric therapy:
Empiric therapy involves the initiation of antimicrobial treatment based on clinical judgment and knowledge of likely pathogens before culture and sensitivity results are available. Culture and sensitivity tests help confirm the causative microorganism and guide subsequent treatment decisions, including adjustments to therapy based on the results. Therefore, while culture and sensitivity tests inform decisions regarding antimicrobial therapy, they do not directly determine whether empiric therapy should be initiated.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
Correct Answer is C
Explanation
A. Discoloration of body fluids:
Vancomycin can cause discoloration of body fluids, particularly urine, resulting in a brownish discoloration. However, this is not caused by histamine release.
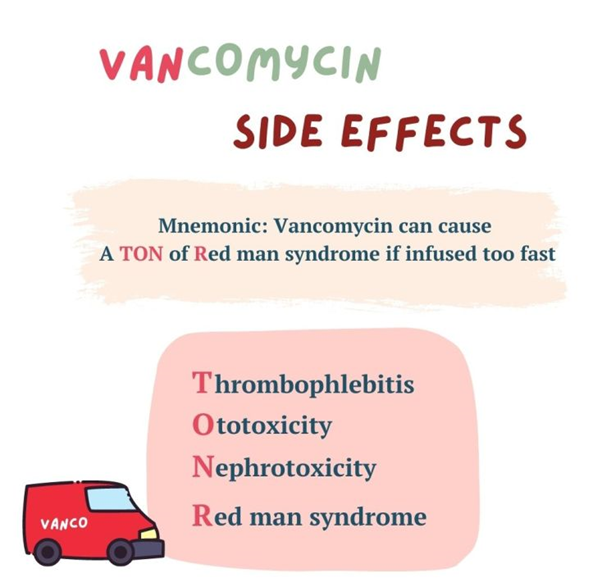
B. Ototoxicity:
Ototoxicity refers to damage to the inner ear structures leading to hearing loss or balance problems. While vancomycin can cause ototoxicity, it is not specifically associated with histamine release.
C. Red-man syndrome
Red-man syndrome, also known as red-neck syndrome or red-person syndrome, is a hypersensitivity reaction characterized by flushing of the skin, particularly the upper body and face, resembling a "red man." This reaction is typically associated with the rapid infusion of vancomycin and is caused by the release of histamine from mast cells and basophils. It is not an allergic reaction but rather a non-immunologic response to vancomycin.
D. Nephrotoxicity:
Nephrotoxicity refers to kidney damage caused by certain medications or toxins. While vancomycin can cause nephrotoxicity, it is not specifically associated with histamine release.
Correct Answer is D
Explanation
A. Sulfonamides:
Sulfonamides are a class of antibiotics that are structurally distinct from cephalosporins like cefazolin. Allergic reactions to sulfonamides do not necessarily indicate a risk of allergy to cefazolin. However, it's still important to assess for any previous allergic reactions to medications, including sulfonamides, as individuals can have multiple medication allergies.
B. Macrolides:
Macrolides are another class of antibiotics that are structurally different from cephalosporins. Allergic reactions to macrolides do not directly indicate an allergy to cefazolin. However, as with sulfonamides, it's crucial to assess for any history of allergic reactions to medications, including macrolides.
C. Yeast:
Yeast is not a class of antibiotics but rather a type of fungus. Allergic reactions to yeast are unrelated to cephalosporin antibiotics like cefazolin. Therefore, a history of allergic reactions to yeast does not suggest an allergy to cefazolin.
D. Penicillin:
This is the correct choice. Penicillins and cephalosporins share a similar beta-lactam ring structure. Individuals who have had allergic reactions to penicillin may have an increased risk of cross-reactivity with cephalosporins, including cefazolin. Therefore, it's crucial to assess for any previous allergic reactions to penicillin before administering cefazolin to avoid potential allergic reactions or adverse effects.
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
