In the following data table of an experiment carried out at 4°C (39.2 F) over 4 hours
|
Solution in bag |
Solution outside bag |
Bag mass change (g): |
|
water |
Water |
-0.2 |
|
20% sucrose |
Water |
+2.4 |
|
, 40% sucrose |
Water |
+4.3 |
|
, 60% sucrose |
water |
+5.8 |
Which of the following options represents the dependent variable?
Duration
Temperature
Bag mass change
Sucrose concentrations.
The Correct Answer is C
In an experiment, the dependent variable is the variable that is being measured and is expected to change in response to changes in the independent variable(s).
In this case, the bag mass change is being measured and is expected to change in response to changes in the independent variable (sucrose concentration).
Choice A is incorrect because duration is not a variable in this experiment.
Choice B is incorrect because temperature is not a variable in this experiment.
Choice D is incorrect because sucrose concentration is an independent variable, not a dependent variable.
An independent variable is a variable that is manipulated by the experimenter to see how it affects the dependent variable.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
Correct Answer is B
Explanation
Diffusion down a concentration gradient causes most of the carbon dioxide from the blood to move into the alveoli.
The alveoli are tiny air sacs in the lungs where gas exchange occurs.
Carbon dioxide is a waste product of cellular respiration and is carried by the blood to the lungs to be exhaled.
In the lungs, carbon dioxide diffuses from the blood (where its concentration is high) into the alveoli (where its concentration is lower) down its concentration gradient.
Choice A is incorrect because carbon dioxide is not converted to carbon monoxide in the body.
Choice C is incorrect because passive transport using carrier proteins is not the primary mechanism by which carbon dioxide moves from the blood into the alveoli.
Choice D is incorrect because active transport using energy is not involved in the movement of carbon dioxide from the blood into the alveoli.
Correct Answer is B
Explanation
The diameter is the measurement of a straight line passing through the centre of a circle and connecting two points on its circumference.
In this case, the line across the center of the cell represents the diameter of the cell.
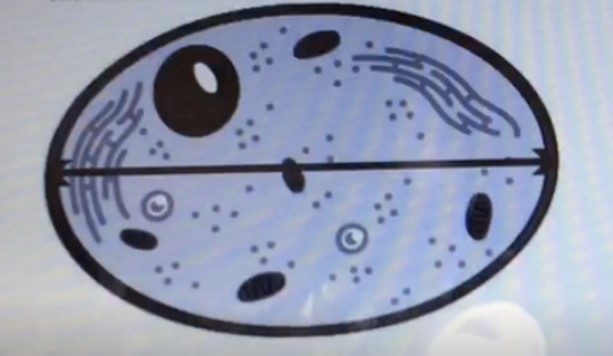
Choice A, Area, is not the correct answer because area refers to the amount of space inside a two-dimensional shape.
Choice C, Volume, is not the correct answer because volume refers to the amount of space occupied by a three-dimensional object.
Choice D, Radius, is not the correct answer because radius refers to the distance from the center of a circle to its circumference and is half the length of the diameter.
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
