The nurse observed that a client with diabetic ketoacidosis is experiencing abnormally deep, regular, and rapid respirations. How would the nurse document this observation in the medical record?
Bradypnea
Kussmaul's respirations
Cheyne-Stokes respirations
Biot's respirations
The Correct Answer is B
Choice A reason: This is incorrect because bradypnea is a term for slow breathing, usually less than 12 breaths per minute. The client is breathing rapidly, not slowly.
Choice B reason: This is correct because Kussmaul's respirations are a type of breathing pattern that is deep, regular, and rapid, usually more than 20 breaths per minute. Kussmaul's respirations are a sign of metabolic acidosis, which occurs in diabetic ketoacidosis due to the accumulation of ketones in the blood. The client is trying to exhale the excess carbon dioxide and lower the acidity of the blood.
Choice C reason: This is incorrect because Cheyne-Stokes respirations are a type of breathing pattern that is irregular, with periods of apnea (no breathing) alternating with periods of rapid breathing. Cheyne-Stokes respirations are a sign of cerebral dysfunction, such as stroke, brain injury, or coma.
Choice D reason: This is incorrect because Biot's respirations are a type of breathing pattern that is irregular, with periods of apnea (no breathing) interspersed with periods of normal breathing. Biot's respirations are a sign of brainstem damage, such as meningitis, encephalitis, or head trauma.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
Correct Answer is A
Explanation
Choice A reason: This is correct because metabolic alkalosis is characterized by a high pH and a high HCO3-. The client's pH and HCO3- are both high, indicating a metabolic disorder. The condition is uncompensated because the PaCO2 is normal, meaning the respiratory system is not compensating for the metabolic alkalosis.
Choice B reason: This is incorrect because metabolic acidosis is characterized by a low pH and a low HCO3-. The client's pH and HCO3- are both high, indicating alkalosis, not acidosis.
Choice C reason: This is incorrect because respiratory alkalosis is characterized by a high pH and a low PaCO2. The client's pH is high, but PaCO2 is normal, indicating a metabolic problem, not a respiratory one.
Choice D reason: This is incorrect because respiratory acidosis is characterized by a low pH and a high PaCO2. The client's pH is high, and PaCO2 is normal, indicating a metabolic problem, not a respiratory one.
Correct Answer is {"dropdown-group-1":"A"}
Explanation
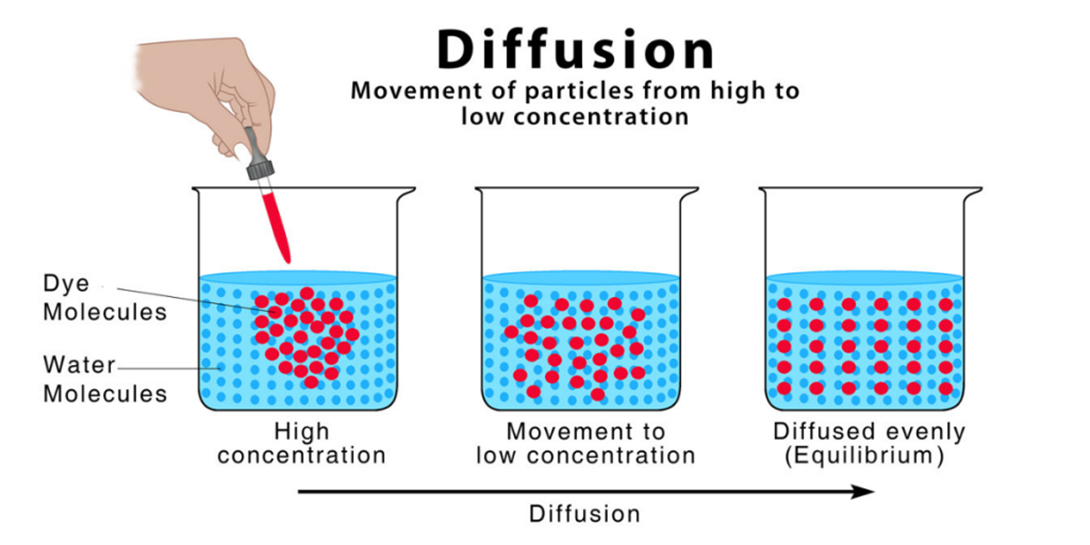
Choice A reason: Diffusion is the process by which small ions such as glucose, oxygen, and carbon dioxide redistribute themselves through semipermeable membranes from areas of higher concentration to areas of lower concentration. This is how these molecules move across the cell membrane and the capillary wall.
Choice B reason: Osmosis is the process by which water moves through semipermeable membranes from areas of lower solute concentration to areas of higher solute concentration. This is how water balance is maintained across the cell membrane and the capillary wall.
Choice C reason: Blood pressure is the force exerted by the blood on the walls of the blood vessels. It is not a process by which small ions redistribute themselves through semipermeable membranes, but rather a factor that influences the movement of fluids and solutes across the capillary wall.
Choice D reason: Rehydration is the process of restoring the fluid balance in the body by drinking fluids or receiving intravenous fluids. It is not a process by which small ions redistribute themselves through semipermeable membranes, but rather a treatment for dehydration.
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
