What is the normal range of serum potassium level in adults?
3.5 to 5.0 mEq/L
8.5 to 10.5 mg/dL
135 to 145 mEq/L
1.8 to 2.6 mEq/L
The Correct Answer is A
Choice A reason: This is correct because 3.5 to 5.0 mEq/L is the normal range of serum potassium level in adults. Potassium is an electrolyte that is important for nerve and muscle function, as well as acid-base balance.
Choice B reason: This is incorrect because 8.5 to 10.5 mg/dL is the normal range of serum calcium level in adults, not potassium. Calcium is an electrolyte that is involved in bone health, muscle contraction, and blood clotting.
Choice C reason: This is incorrect because 135 to 145 mEq/L is the normal range of serum sodium level in adults, not potassium. Sodium is an electrolyte that is essential for fluid balance, nerve transmission, and muscle contraction.
Choice D reason: This is incorrect because 1.8 to 2.6 mEq/L is the normal range of serum magnesium level in adults, not potassium. Magnesium is an electrolyte that is important for muscle and nerve function, as well as enzyme activity.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
Correct Answer is A
Explanation
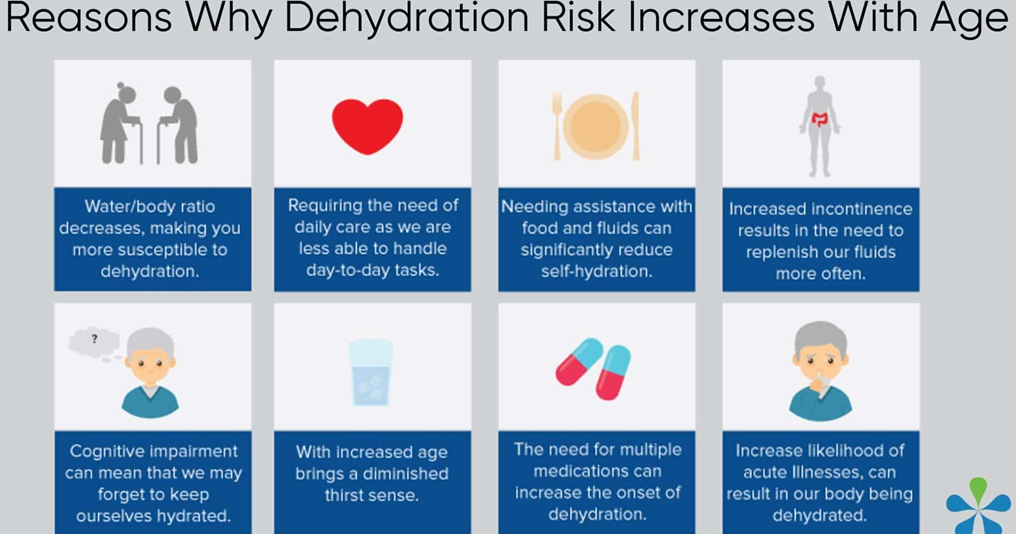
Choice A: Elderly patients are at a higher risk for dehydration due to physiological changes that come with aging, such as decreased kidney function and physical changes to the body's water balance systems. Additionally, fever increases metabolic rate and fluid loss, and nausea and vomiting prevent adequate fluid intake, further increasing the risk of dehydration.
Choice B: While intentionally limiting fluid intake can lead to dehydration, the body's thirst mechanism in a healthy teenager is typically strong enough to prevent severe dehydration.
Choice C: Diarrhea can certainly lead to dehydration, but a young, otherwise healthy patient typically has a stronger ability to recover from fluid loss than an elderly patient.
Choice D: Infants are at a higher risk for dehydration than older children and adults due to their smaller body weight and higher turnover of water and electrolytes, but in this case, the elderly patient's multiple risk factors put them at a higher risk overall.
Correct Answer is A
Explanation
Choice A reason: This is correct because acidosis is a condition where the serum pH is lower than the normal range of 7.35 to 7.45. Acidosis can be caused by an excess of acids or a loss of bases in the body, which can affect the function of various organs and systems.
Choice B reason: This is incorrect because equal bicarbonate is not a condition, but a term that describes the balance between bicarbonate (HCO3-) and carbonic acid (H2CO3) in the blood. Bicarbonate is a base that buffers the acids in the blood and maintains the pH. Equal bicarbonate means that the ratio of bicarbonate to carbonic acid is 20:1, which is the normal value.
Choice C reason: This is incorrect because neutral carbonic acid is not a condition, but a term that describes the pH of carbonic acid (H2CO3) in the blood. Carbonic acid is an acid that forms when carbon dioxide (CO2) dissolves in water. Neutral carbonic acid means that the pH of carbonic acid is 7.0, which is neither acidic nor basic.
Choice D reason: This is incorrect because alkalosis is a condition where the serum pH is higher than the normal range of 7.35 to 7.45. Alkalosis can be caused by a loss of acids or an excess of bases in the body, which can affect the function of various organs and systems.
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
