When testing tonicity in Elodea cells, once Elodea cells were placed in distilled water, what did you observe? Select all that apply.
Cell membranes pressed tightly against the cell walls.
Many of the cells had burst.
Full central vacuoles.
Many of the cells had become crenated.
Correct Answer : A,C
Choice A rationale: Cell membranes pressed tightly against the cell walls is correct because this is what happens when a plant cell is placed in a hypotonic solution. A hypotonic solution has a higher concentration of water than the cell, so water moves into the cell and out of the solution by osmosis, causing the cell to swell and press against the cell wall. This is called turgor and it helps the cell maintain its shape and rigidity.
Choice B rationale: Many of the cells had burst is incorrect because plant cells do not burst in a hypotonic solution, unlike animal cells. Plant cells have a rigid cell wall that prevents them from bursting, even when they are full of water. The cell wall can withstand the pressure of water entering the cell.
Choice C rationale: Full central vacuoles is correct because this is also what happens when a plant cell is placed in a hypotonic solution. The central vacuole is a large organelle that stores water and other substances in the plant cell. When water enters the cell, the central vacuole expands and fills up the cell, increasing its turgor pressure.
Choice D rationale: Many of the cells had become crenated is incorrect because crenation is the opposite of what happens in a hypotonic solution. Crenation is the process by which a cell shrinks and becomes wrinkled due to water loss in a hypertonic solution. A hypertonic solution has a lower concentration of water than the cell, so water moves out of the cell and into the solution by osmosis, causing the cell to shrink.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
Correct Answer is {"dropdown-group-1":"C"}
Explanation
Choice A rationale: Osmotic is incorrect because osmotic is an adjective that describes the movement of water across a semipermeable membrane, not a type of solution. Osmosis is the process by which water moves from a region of high water concentration to a region of low water concentration.
Choice B rationale: Isotonic is incorrect because isotonic is a type of solution that has the same concentration of water as the cell placed in the solution. In an isotonic solution, there is no net movement of water across the cell membrane.
Choice C rationale: Hypertonic is correct because hypertonic is a type of solution that has a lower concentration of water than the cell placed in the solution. In a hypertonic solution, water moves out of the cell and into the solution by osmosis, causing the cell to shrink.
Choice D rationale: Diffusive is incorrect because diffusive is an adjective that describes the movement of molecules from a region of high concentration to a region of low concentration, not a type of solution. Diffusion is the process by which molecules move across a membrane or a space due to their random motion.
Choice E rationale: Hypotonic is incorrect because hypotonic is a type of solution that has a higher concentration of water than the cell placed in the solution. In a hypotonic solution, water moves into the cell and out of the solution by osmosis, causing the cell to swell.
Correct Answer is B
Explanation
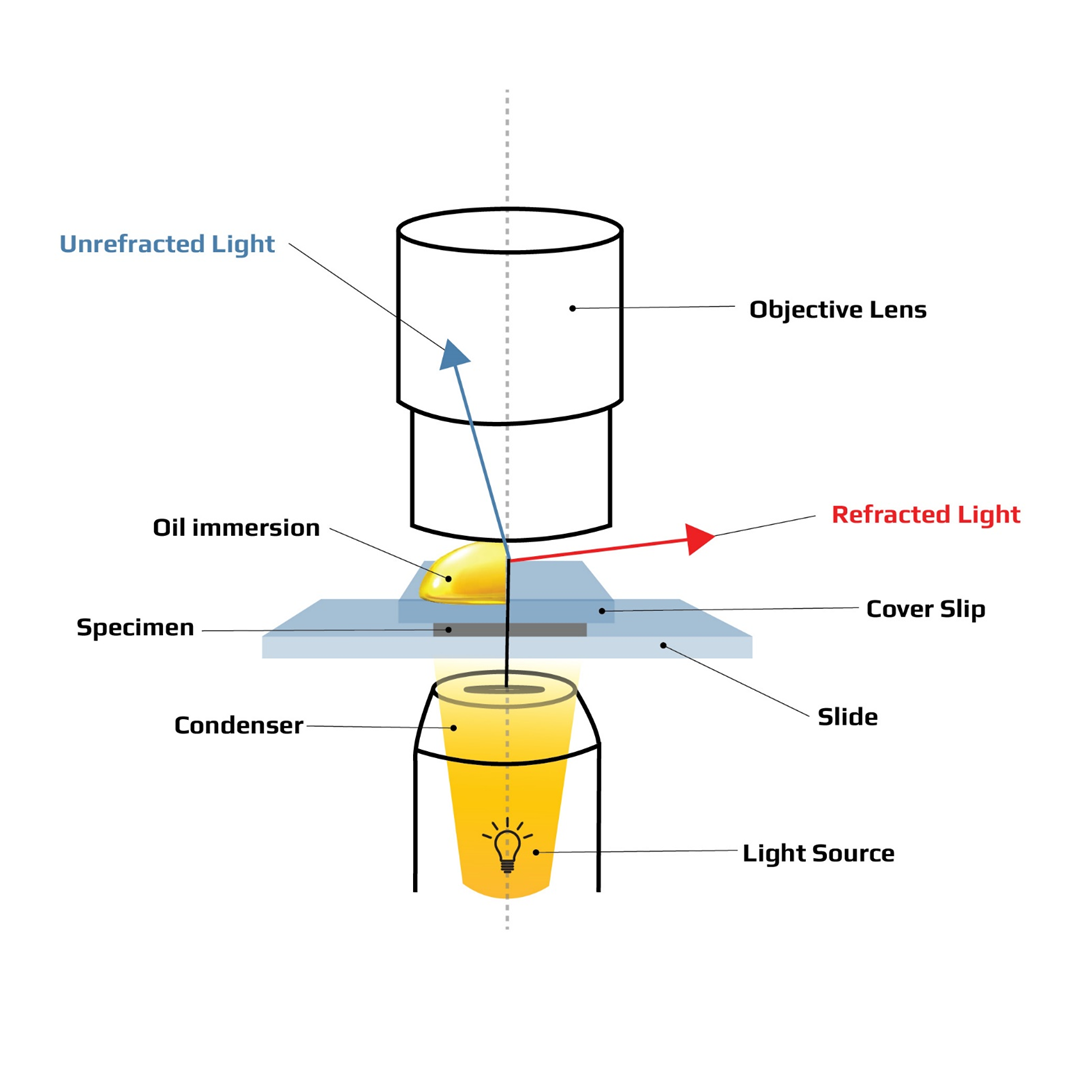
Choice A rationale: High power is incorrect because high power is the second highest magnification objective lens, not the highest. High power is also called the 40x objective lens because it magnifies the specimen by 40 times. When combined with the 10x eyepiece lens, the total magnification is 400x.
Choice B rationale: Oil immersion is correct because oil immersion is the highest magnification objective lens. Oil immersion is also called the 100x objective lens because it magnifies the specimen by 100 times. When combined with the 10x eyepiece lens, the total magnification is 1000x. Oil immersion requires oil to be applied between the slide and the lens to reduce the refraction of light and increase the clarity of the image.
Choice C rationale: Low power is incorrect because low power is the second lowest magnification objective lens, not the highest. Low power is also called the 10x objective lens because it magnifies the specimen by 10 times. When combined with the 10x eyepiece lens, the total magnification is 100x.
Choice D rationale: Scanning is incorrect because scanning is the lowest magnification objective lens, not the highest. Scanning is also called the 4x objective lens because it magnifies the specimen by 4 times. When combined with the 10x eyepiece lens, the total magnification is 40x. Scanning is used to scan the whole slide and find the specimen.
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
