Which of the following ions binds to the troponin complex, initiating muscle contraction?
Potassium.
Calcium.
Phosphorus.
Sodium.
The Correct Answer is B
Calcium ions play a crucial role in initiating muscle contraction.
When a muscle cell is stimulated to contract by an action potential, calcium channels open in the sarcoplasmic membrane and release calcium into the sarcoplasm.
Some of this calcium attaches to troponin, which causes it to change shape. This shape change exposes binding sites for myosin on the actin filaments.
Myosin’s binding to actin causes cross-bridge formation, and muscle contraction begins.
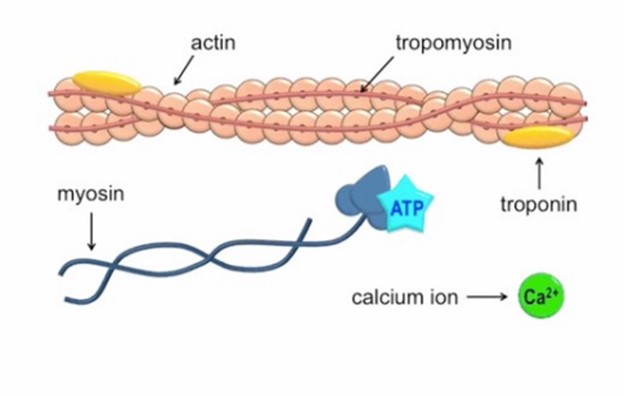
The other ions mentioned in the question do not have this specific role in muscle contraction.
Potassium ions are important for maintaining the resting membrane potential of cells, but they do not bind to the troponin complex.
Phosphorus ions are important for energy metabolism but do not bind to the troponin complex.
Sodium ions are important for generating action potentials but do not bind to the troponin complex.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
Correct Answer is C
Explanation
In an experiment, the dependent variable is the variable that is being measured and is expected to change in response to changes in the independent variable(s).
In this case, the bag mass change is being measured and is expected to change in response to changes in the independent variable (sucrose concentration).
Choice A is incorrect because duration is not a variable in this experiment.
Choice B is incorrect because temperature is not a variable in this experiment.
Choice D is incorrect because sucrose concentration is an independent variable, not a dependent variable.
An independent variable is a variable that is manipulated by the experimenter to see how it affects the dependent variable.
Correct Answer is A
Explanation
As a solid turns to a liquid, the particles become less ordered and more free to move around.
Choice B is not correct because particles have an increase in mobility as a solid turns to a liquid.
Choice C is not correct because particles move further apart as a solid turns to a liquid.
Choice D is not correct because intermolecular forces between particles become weaker as a solid turns to a liquid.
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
