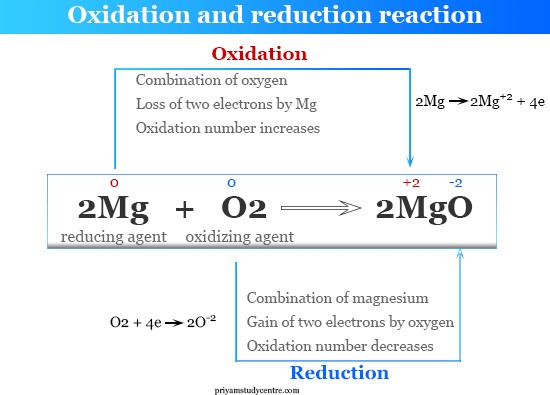
Which of the following occurs in an oxidation reaction?
Removal of oxygen
Addition of carbon
Addition of neutrons
Removal of electrons
The Correct Answer is D
An oxidation reaction occurs when there is a removal of electrons. Oxidation is the loss of electrons during a reaction by a molecule, atom, or ion. When oxidation occurs, the oxidation state of the chemical species increases.
The other options are not correct because they do not accurately describe what occurs in an oxidation reaction. Removal of oxygen, addition of carbon, and addition of neutrons are not processes that occur in an oxidation reaction.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
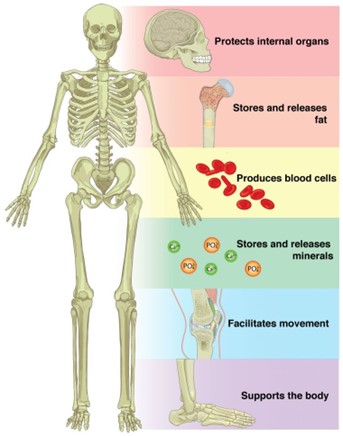
Correct Answer is A
Explanation
Storage of minerals. Bones serve as a storage site for minerals such as calcium and phosphorus. These minerals are essential for various bodily functions and can be released from the bones into the bloodstream when needed.
B. Detoxification of alcohol is not a function of bone. This process occurs primarily in the liver.
C. Secretion of hormones is not a function of bone. Hormones are produced and secreted by glands such as the pituitary gland, thyroid gland, and adrenal glands.
D. Production of otoliths is not a function of bone. Otoliths are small calcium carbonate structures found in the inner ear of fish and other vertebrates that help with balance and hearing.
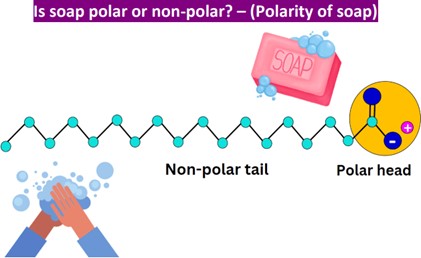
Correct Answer is A
Explanation
Soap’s dual polar and nonpolar nature helps bond oil and water. Soap is an emulsifier, which means that it has both polar and nonpolar regions. The polar regions of soap molecules are attracted to water, while the nonpolar regions are attracted to oil and grease. This allows soap to bond with both water and oil, helping to remove dirt and grime from surfaces.
B. Soap’s acidity does not cause grime to precipitate into the water.
C. Soap does not have enzymatic action that helps to dissolve grime into small particles.
D. Soap’s texture does not physically scour grime off surfaces.
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
