Which of the following best describes the result of using a catalyst in a chemical reaction?
The reaction is completed in a shorter amount of time.
A more desirable product is often formed.
A greater amount of heat energy is released by the reaction.
The yield of product is increased.
The Correct Answer is A
A catalyst is a substance that increases the rate of a chemical reaction without being consumed by the reaction.
As a result, the reaction is completed in a shorter amount of time.
Choice B is not correct because using a catalyst does not necessarily result in the formation of a more desirable product.
Choice C is not correct because using a catalyst does not necessarily result in the release of a greater amount of heat energy by the reaction.
Choice D is not correct because using a catalyst does not necessarily increase the yield of product.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
Correct Answer is C
Explanation
During ovulation, a mature egg is released from the female ovary, enabling it to be fertilized by male sperm cells.
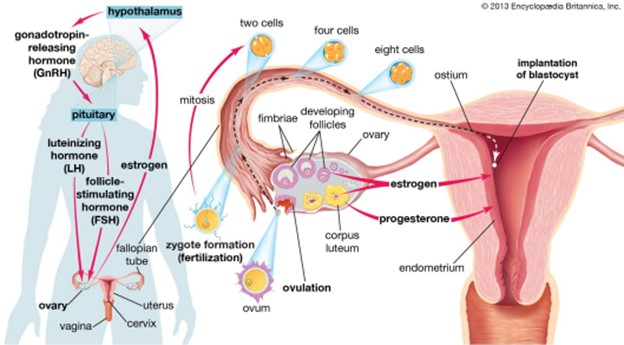
Choice A is incorrect because menstruation is the process of shedding the uterine lining, which occurs when an egg is not fertilized.
Choice B is incorrect because fertilization is the process of a sperm cell joining with an egg cell to form a zygote.
Choice D is incorrect because oogenesis is the process of forming female gametes (eggs) in the ovaries.
Correct Answer is D
Explanation
Viruses lack the essential machinery needed to reproduce by themselves.
In fact, viruses can only reproduce after infecting a living cell - a process called viral replication.
Once inside a living cell, viruses re-program the cell’s machinery to produce viral proteins and genetic material to make new copies of themselves.
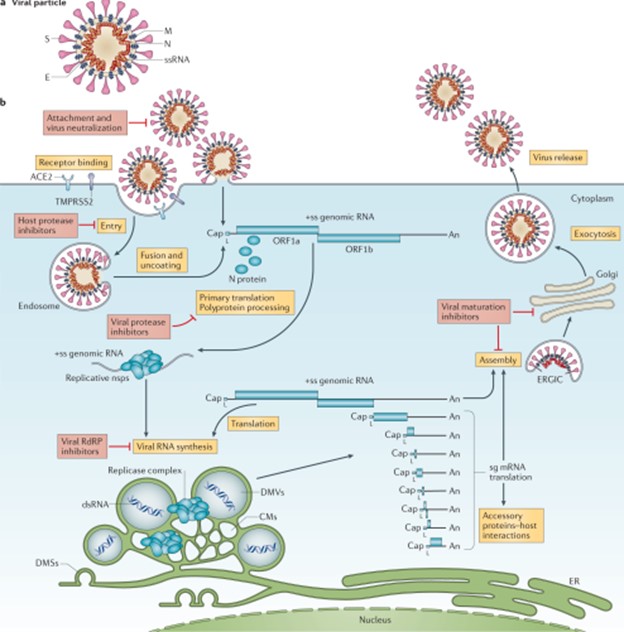
Choice A, Bacteria, is not the correct answer because bacteria have their own metabolic pathways and can reproduce outside of a host cell.
Choice B, Protozoa, is also not the correct answer because protozoa are single-celled eukaryotes that have their own metabolic pathways and can reproduce outside of a host cell.
Choice C, Helminths, is not the correct answer because helminths are multicellular parasitic worms that have their own metabolic pathways and can reproduce outside of a host cell.
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
