Which of the following properties of water explains its solvent abilities for certain substances?
Kinetic energy of liquid water molecules
High specific heat
High surface tension
Polarity of water molecules.
The Correct Answer is D
The polarity of water molecules explains its solvent abilities for certain substances.
Water is a polar molecule because it has a partial positive charge on one end and a partial negative charge on the other end due to the unequal sharing of electrons between the oxygen and hydrogen atoms.
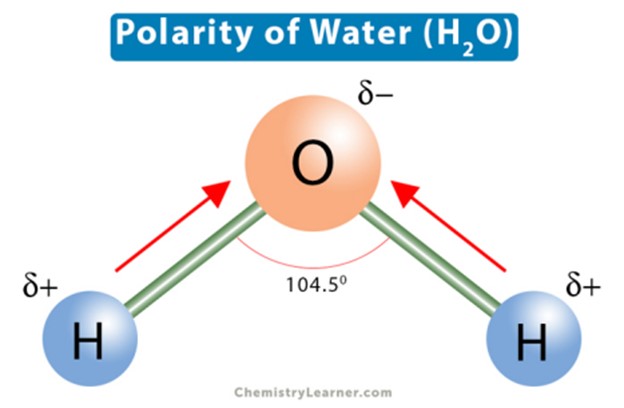
This polarity allows water to dissolve other polar substances and ionic compounds.
Choice A. Kinetic energy of liquid water molecules is not the correct answer because kinetic energy refers to the energy of motion and does not directly explain water’s solvent abilities.
Choice B. High specific heat is not the correct answer because specific heat refers to the amount of heat required to raise the temperature of a substance and does not directly explain water’s solvent abilities.
Choice C. High surface tension is not the correct answer because surface tension refers to the cohesive forces between liquid molecules and does not directly explain water’s solvent abilities.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
Correct Answer is C
Explanation
A control group is a group in an experiment that does not receive the treatment or manipulation being tested and is used as a benchmark to measure how the other tested subjects do.
The control group is used to minimize the effects of all variables except the independent variable.
This allows researchers to determine if changes in the dependent variable are due to the manipulation of the independent variable or if they are due to some other factor.
Choice A. Responding is not the correct answer because it refers to the dependent variable, which is the variable that is being measured in an experiment.
Choice B. Manipulated is not the correct answer because it refers to the independent variable, which is the variable that is being manipulated in an experiment.
Choice D. Variable is not the correct answer because it refers to any factor that can change in an experiment and can include both independent and dependent variables.
Correct Answer is D
Explanation
When viruses cause an infection, they spread their DNA, affecting healthy cells’ genetic makeup and potentially causing them to turn into cancer.
For instance, HPV infections cause the virus’ DNA to combine with the host’s DNA, disrupting the normal function of cells.
Choice A is not correct because cancerous and normal cells sharing genetic sequences do not support the hypothesis that viruses can cause cancer.
Choice B is not correct because cellular DNA having sequences related to viral sequences does not support the hypothesis that viruses can cause cancer.
Choice C is not correct because viruses and cancer cells both replicating rapidly do not support the hypothesis that viruses can cause cancer.
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
