What is the approximate threshold value for mammalian neurons?
-55 mV.
-80 mV.
+35 mV.
0 mV.
The Correct Answer is A
The threshold potential is the critical level to which a membrane potential must be depolarized to initiate an action potential.
Most often, the threshold potential is a membrane potential value between –50 and –55 mV.
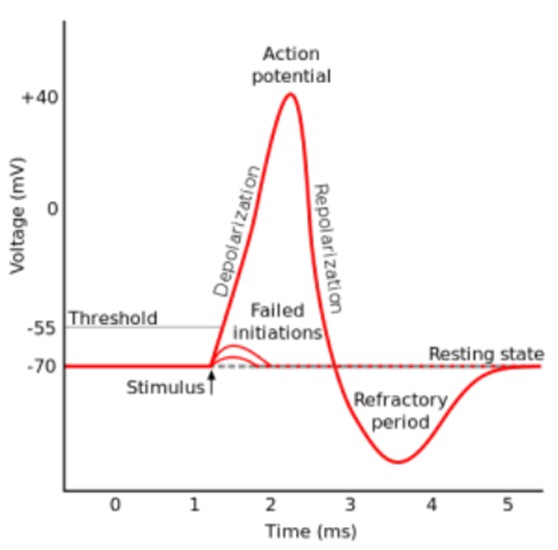
The membrane potential of a neuron is determined by the distribution of ions across the cell membrane.
At rest, the inside of a neuron is more negative than the outside due to the presence of negatively charged proteins and other molecules.
The movement of ions across the cell membrane can change the membrane potential.
For example, when sodium ions enter the cell, they make the inside of the cell more positive (less negative), causing depolarization.
Choice B is incorrect because -80 mV is below the typical threshold value for mammalian neurons.
Choice C is incorrect because +35 mV is above the typical threshold value for mammalian neurons.
Choice D is incorrect because 0 mV is above the typical threshold value for mammalian neurons.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
Correct Answer is D
Explanation
The pleura is a double-layered serous membrane that covers each lung and lines the thoracic cage.
The pleura is a vital part of the respiratory tract.
Its role is to cushion the lung and reduce any friction that may develop between the lung, rib cage, and chest cavity.
Each pleura (there are two) consists of a two-layered membrane that covers each lung.
The layers are separated by a small amount of viscous (thick) lubricant known as pleural fluid.
The pleura is comprised of two distinct layers: the visceral pleura and the parietal pleura.
The visceral pleura is the thin, slippery membrane that covers the surface of the lungs and dips into the areas separating the different lobes of the lungs (called the hilum).
Correct Answer is B
Explanation
Calcium ions play a crucial role in initiating muscle contraction.
When a muscle cell is stimulated to contract by an action potential, calcium channels open in the sarcoplasmic membrane and release calcium into the sarcoplasm.
Some of this calcium attaches to troponin, which causes it to change shape. This shape change exposes binding sites for myosin on the actin filaments.
Myosin’s binding to actin causes cross-bridge formation, and muscle contraction begins.
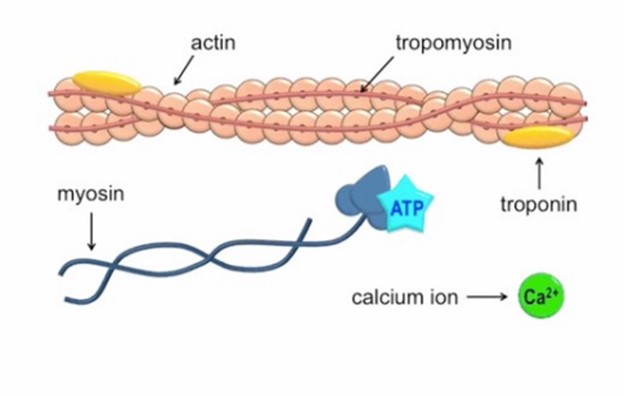
The other ions mentioned in the question do not have this specific role in muscle contraction.
Potassium ions are important for maintaining the resting membrane potential of cells, but they do not bind to the troponin complex.
Phosphorus ions are important for energy metabolism but do not bind to the troponin complex.
Sodium ions are important for generating action potentials but do not bind to the troponin complex.
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
