Which of the following tests requires heating prior to observing results?
Benedict's test
Brown paper test
Biuret test
Iodine test
Wendelspecht test
The Correct Answer is A
Choice A reason: Benedict's test is a test for the presence of reducing sugars, such as glucose or maltose, in a solution. The test involves adding Benedict's reagent, which is a blue solution of copper (II) sulfate, sodium carbonate, and sodium citrate, to the solution and heating it in a water bath. If reducing sugars are present, they reduce the copper (II) ions to copper (I) ions, which form a red, orange, or green precipitate of copper (I) oxide. The color and amount of the precipitate indicate the concentration of reducing sugars in the solution. ¹
Choice B reason: Brown paper test is a test for the presence of lipids, such as fats or oils, in a solution. The test involves placing a drop of the solution on a piece of brown paper and letting it dry. If lipids are present, they leave a translucent spot on the paper, which can be seen by holding the paper against a light source. The test is based on the fact that lipids are nonpolar and do not dissolve in water, but can dissolve in organic solvents and stain the paper. ²
Choice C reason: Biuret test is a test for the presence of proteins or peptides in a solution. The test involves adding Biuret reagent, which is a blue solution of copper (II) sulfate and sodium hydroxide, to the solution. If proteins or peptides are present, they form a complex with the copper (II) ions, which changes the color of the solution to violet or pink. The test is based on the fact that proteins and peptides have peptide bonds, which have nitrogen atoms that can coordinate with the copper (II) ions. ³
Choice D reason: Iodine test is a test for the presence of starch in a solution. The test involves adding iodine solution, which is a brown solution of iodine and potassium iodide, to the solution. If starch is present, it forms a complex with the iodine molecules, which changes the color of the solution to blue-black. The test is based on the fact that starch is a polysaccharide that has a helical structure, which can trap the iodine molecules inside. ⁴
Choice E reason: Wendelspecht test is a fictional test that does not exist in reality. It is a made-up name that has no meaning or relevance to the topic of this question. Therefore, it cannot be a valid answer.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
Correct Answer is A
Explanation
Choice A rationale: Movable is correct because motile is an adjective that describes the ability of an organism or a cell to move by itself, using some form of locomotion, such as flagella, cilia, or pseudopodia. For example, some bacteria, protists, and sperm cells are motile.
Choice B rationale: Fixed is incorrect because fixed is the opposite of motile. Fixed is an adjective that describes the inability of an organism or a cell to move by itself, due to being attached to a substrate or a surface. For example, some plants, fungi, and coral are fixed.
Choice C rationale: Magnification is incorrect because magnification is not related to motility. Magnification is a measure of how much larger an image appears compared to the actual size of the object. Magnification is used in microscopy to observe small objects, such as cells.
Choice D rationale: Solid is incorrect because solid is not related to motility. Solid is a state of matter that has a definite shape and volume, due to the strong attraction between the molecules. Solid is contrasted with liquid and gas, which have no definite shape and can flow.
Correct Answer is B
Explanation
Choice A rationale: High power is incorrect because high power is the second highest magnification objective lens, not the highest. High power is also called the 40x objective lens because it magnifies the specimen by 40 times. When combined with the 10x eyepiece lens, the total magnification is 400x.
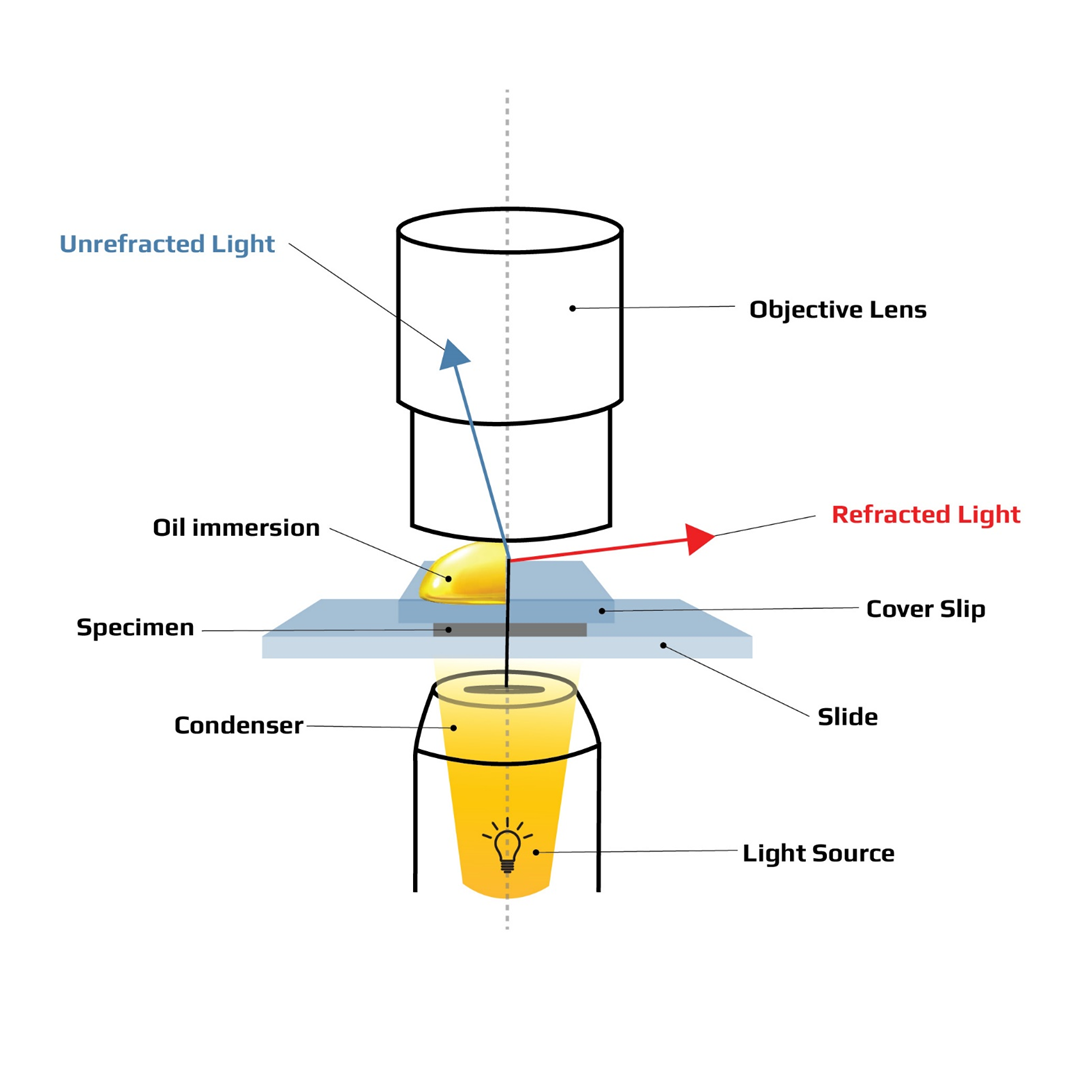
Choice B rationale: Oil immersion is correct because oil immersion is the highest magnification objective lens. Oil immersion is also called the 100x objective lens because it magnifies the specimen by 100 times. When combined with the 10x eyepiece lens, the total magnification is 1000x. Oil immersion requires oil to be applied between the slide and the lens to reduce the refraction of light and increase the clarity of the image.
Choice C rationale: Low power is incorrect because low power is the second lowest magnification objective lens, not the highest. Low power is also called the 10x objective lens because it magnifies the specimen by 10 times. When combined with the 10x eyepiece lens, the total magnification is 100x.
Choice D rationale: Scanning is incorrect because scanning is the lowest magnification objective lens, not the highest. Scanning is also called the 4x objective lens because it magnifies the specimen by 4 times. When combined with the 10x eyepiece lens, the total magnification is 40x. Scanning is used to scan the whole slide and find the specimen.
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
