Fluid and electrolyte balance is maintained through the process of fluid and solutes moving in and out of cells. What specific process allows fluid to pass through a membrane from a dilute to a more concentrated area?
Active transport
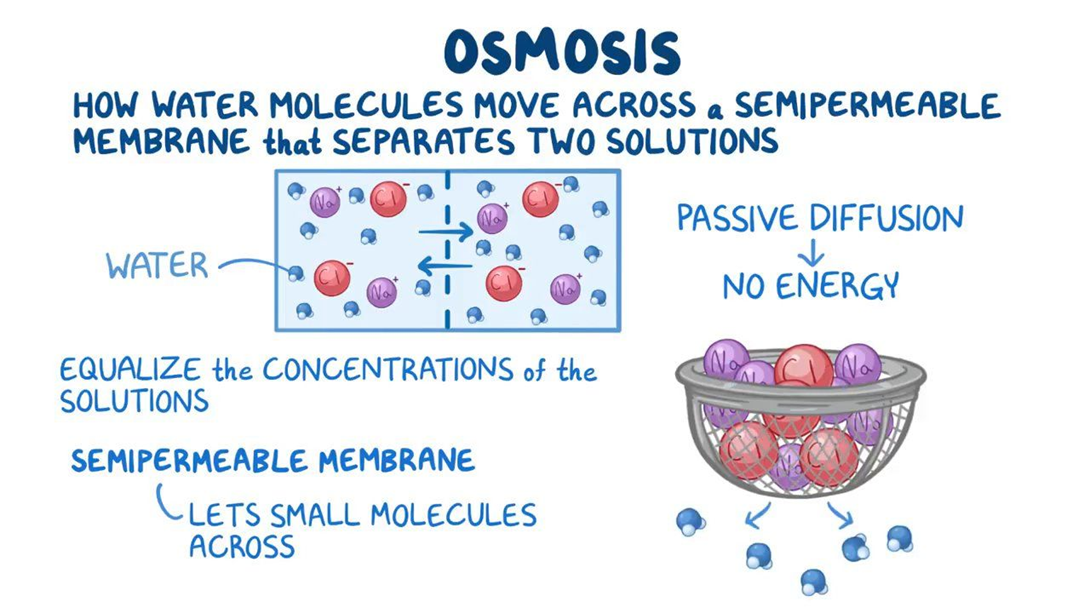
Osmosis
Filtration
Diffusion
The Correct Answer is B
Choice A reason: Active transport is not the process that allows fluid to pass through a membrane from a dilute to a more concentrated area. Active transport is the process that moves solutes across a membrane against their concentration gradient, using energy from ATP. Active transport can create or maintain a concentration difference between two sides of a membrane.
Choice B reason: Osmosis is the process that allows fluid to pass through a membrane from a dilute to a more concentrated area. Osmosis is the movement of water across a selectively permeable membrane from an area of low solute concentration to an area of high solute concentration. Osmosis can equalize the concentration of solutes on both sides of a membrane.
Choice C reason: Filtration is not the process that allows fluid to pass through a membrane from a dilute to a more concentrated area. Filtration is the movement of fluid and solutes across a membrane due to a pressure difference between two sides of a membrane. Filtration can separate solutes from fluid based on their size and charge.
Choice D reason: Diffusion is not the process that allows fluid to pass through a membrane from a dilute to a more concentrated area. Diffusion is the movement of solutes across a membrane from an area of high solute concentration to an area of low solute concentration. Diffusion can also equalize the concentration of solutes on both sides of a membrane.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
Correct Answer is C
Explanation
Choice A reason: Pyuria, or pus in the urine, is not a direct sign of fluid volume overload. It may indicate a urinary tract infection, kidney stones, or other renal problems.
Choice B reason: Weight loss is not a typical finding of fluid volume overload. In fact, weight gain is a common symptom of fluid retention, as the body holds more fluid than it excretes.
Choice C reason: Jugular vein distention, or the bulging of the neck veins, is a serious indicator of fluid volume overload. It reflects increased pressure in the right side of the heart and the systemic circulation. It may also signal heart failure, pulmonary hypertension, or pericardial tamponade.
Choice D reason: Muscle contractions are not directly related to fluid volume overload. They may be caused by electrolyte imbalances, dehydration, muscle fatigue, or nerve disorders.
Correct Answer is D
Explanation
Choice A reason: Excessive stomach acid secretion is not the correct answer because it is not a diagnostic test, but a possible cause of peptic ulcer disease. Peptic ulcers are sores that develop in the lining of the stomach or duodenum due to damage from stomach acid and digestive enzymes.
Choice B reason: An incompetent pyloric sphincter is not the correct answer because it is not a diagnostic test, but a possible complication of peptic ulcer disease. The pyloric sphincter is a muscular valve that controls the passage of food from the stomach to the small intestine. If it becomes damaged or weakened, it can cause gastric outlet obstruction, which is a blockage of the stomach.
Choice C reason: A metabolic acid-base imbalance is not the correct answer because it is not a diagnostic test, but a possible consequence of peptic ulcer disease. Peptic ulcers can cause bleeding, perforation, or gastric outlet obstruction, which can affect the acid-base balance of the body. For example, vomiting can cause metabolic alkalosis, which is a condition where the blood is too alkaline.
Choice D reason: An infection with Helicobacter pylori is the correct answer. Helicobacter pylori is a type of bacteria that can infect the stomach and duodenum and cause inflammation and ulcers. It is the most common cause of peptic ulcer disease. The health care provider can order a diagnostic test to detect the presence of Helicobacter pylori in the client's stomach or blood, such as a urea breath test, a stool antigen test, or a blood antibody test.
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
