Nitrogen gas is an extremely stable molecule because of which of the following?
Ionic bonds.
Hydrogen bonds.
Resonance bonds.
Triple covalent bonds.
The Correct Answer is D
Nitrogen gas (N2) is an extremely stable molecule because it consists of two nitrogen atoms bonded together by a triple covalent bond.
A covalent bond is a type of chemical bond where atoms share electrons to form a molecule.
In a triple covalent bond, three pairs of electrons are shared between the two atoms, resulting in a very strong bond that makes the molecule extremely stable.
Choice A. Ionic bonds is not correct because ionic bonds involve the transfer of electrons from one atom to another to form ions, which are then attracted to each other due to their opposite charges.
Nitrogen gas does not contain ions and is not held together by ionic bonds.
Choice B. Hydrogen bonds is not correct because hydrogen bonds are weak electrostatic attractions between molecules that contain hydrogen atoms bonded to highly electronegative atoms such as oxygen or nitrogen.
Nitrogen gas does not contain hydrogen atoms and is not held together by hydrogen bonds.
Choice C. Resonance bonds is not correct because resonance refers to the delocalization of electrons in a molecule where multiple Lewis structures can be drawn to represent the molecule.
Nitrogen gas has a single Lewis structure and does not exhibit resonance.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
Correct Answer is B
Explanation
Electrophoresis is a technique that uses an electric field to separate charged molecules, such as DNA fragments, based on their size and charge.
Choice A is not correct because titration is a laboratory method used to determine the concentration of a solution.
Choice C is not correct because filtration is a laboratory method used to separate solids from liquids.
Choice D is not correct because spectrophotometry is a laboratory method used to measure the absorbance of light by a solution.
Correct Answer is B
Explanation
The diameter is the measurement of a straight line passing through the centre of a circle and connecting two points on its circumference.
In this case, the line across the center of the cell represents the diameter of the cell.
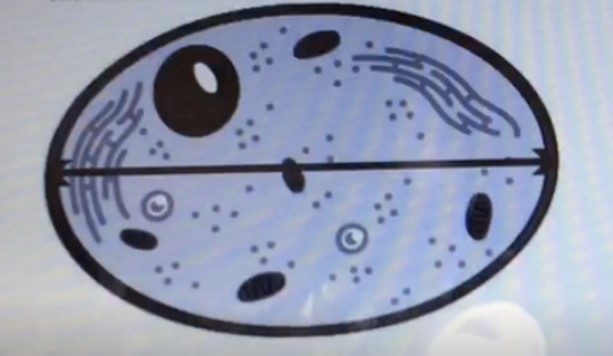
Choice A, Area, is not the correct answer because area refers to the amount of space inside a two-dimensional shape.
Choice C, Volume, is not the correct answer because volume refers to the amount of space occupied by a three-dimensional object.
Choice D, Radius, is not the correct answer because radius refers to the distance from the center of a circle to its circumference and is half the length of the diameter.
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
