Which electrolyte imbalance is most likely to cause abdominal pain, urinary retention, and confusion?
Sodium (Na+)
Calcium (Ca2+)
Chloride (Cl-)
Phosphates (PO4^3^-)
Potassium (K+)
The Correct Answer is B
Choice A reason: Sodium (Na+) imbalance can cause neurological symptoms such as confusion, seizures, or coma, but not abdominal pain or urinary retention.
Choice B reason: Calcium (Ca2+) imbalance can cause abdominal pain, urinary retention, and confusion, as well as muscle weakness, bone pain, and cardiac arrhythmias. These signs are the result of an inadequate supply of calcium, which is essential for nerve and muscle function, as well as bone health.
Choice C reason: Chloride (Cl-) imbalance can cause acid-base disorders such as metabolic acidosis or alkalosis, but not abdominal pain, urinary retention, or confusion.
Choice D reason: Phosphates (PO4^3^-) imbalance can cause bone and muscle problems, such as rickets, osteomalacia, or tetany, but not abdominal pain, urinary retention, or confusion.
Choice E reason: Potassium (K+) imbalance can cause cardiac and neuromuscular symptoms, such as arrhythmias, palpitations, muscle weakness, or paralysis, but not abdominal pain, urinary retention, or confusion.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
Correct Answer is A
Explanation
Choice A reason: This is correct because 3.5 to 5.0 mEq/L is the normal range of serum potassium level in adults. Potassium is an electrolyte that is important for nerve and muscle function, as well as acid-base balance.
Choice B reason: This is incorrect because 8.5 to 10.5 mg/dL is the normal range of serum calcium level in adults, not potassium. Calcium is an electrolyte that is involved in bone health, muscle contraction, and blood clotting.
Choice C reason: This is incorrect because 135 to 145 mEq/L is the normal range of serum sodium level in adults, not potassium. Sodium is an electrolyte that is essential for fluid balance, nerve transmission, and muscle contraction.
Choice D reason: This is incorrect because 1.8 to 2.6 mEq/L is the normal range of serum magnesium level in adults, not potassium. Magnesium is an electrolyte that is important for muscle and nerve function, as well as enzyme activity.
Correct Answer is {"dropdown-group-1":"A"}
Explanation
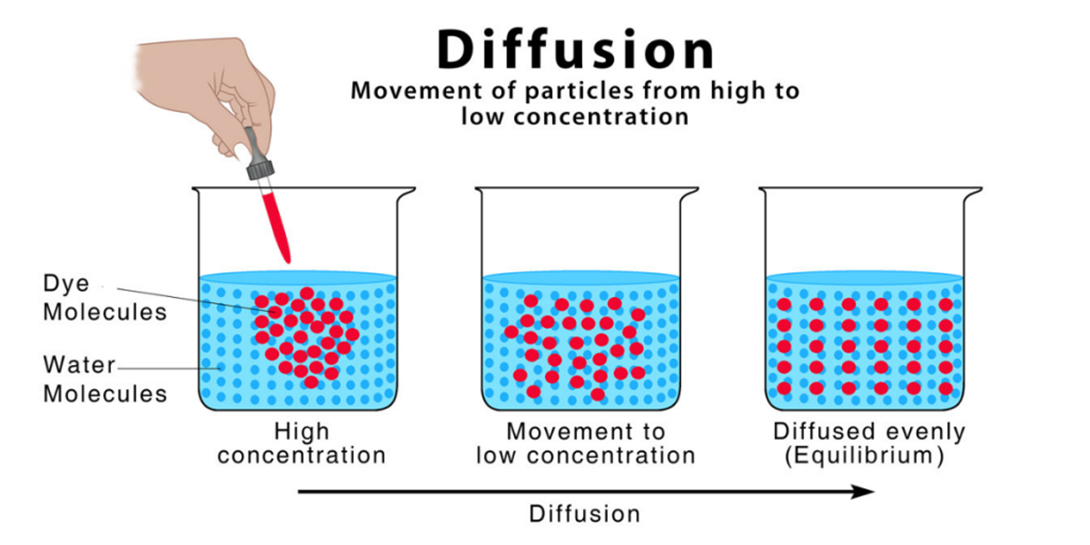
Choice A reason: Diffusion is the process by which small ions such as glucose, oxygen, and carbon dioxide redistribute themselves through semipermeable membranes from areas of higher concentration to areas of lower concentration. This is how these molecules move across the cell membrane and the capillary wall.
Choice B reason: Osmosis is the process by which water moves through semipermeable membranes from areas of lower solute concentration to areas of higher solute concentration. This is how water balance is maintained across the cell membrane and the capillary wall.
Choice C reason: Blood pressure is the force exerted by the blood on the walls of the blood vessels. It is not a process by which small ions redistribute themselves through semipermeable membranes, but rather a factor that influences the movement of fluids and solutes across the capillary wall.
Choice D reason: Rehydration is the process of restoring the fluid balance in the body by drinking fluids or receiving intravenous fluids. It is not a process by which small ions redistribute themselves through semipermeable membranes, but rather a treatment for dehydration.
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
