Which of the following types of cells produce and release antibodies?
Natural killer cells.
Cytotoxic T-cells.
Plasma B cells.
Helper T-cells.
The Correct Answer is C
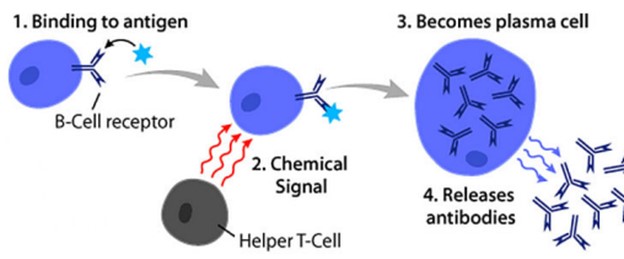
Antibodies are produced by specialized white blood cells called B lymphocytes (or B cells).
When an antigen binds to the B-cell surface, it stimulates the B cell to divide and mature into a group of identical cells called a clone.
The mature B cells, called plasma cells, secrete millions of antibodies into the bloodstream and lymphatic system.
Choice A, Natural killer cells, is not the correct answer because natural killer cells are a type of white blood cell that play a major role in the host-rejection of both tumours and virally infected cells.
Choice B, Cytotoxic T-cells, is not the correct answer because cytotoxic T-cells are a type of white blood cell that kills cancer cells, cells that are infected (particularly with viruses), or cells that are damaged in other ways.
Choice D, Helper T-cells, is not the correct answer because helper T-cells are a type of white blood cell that plays an important role in the immune system by helping other white blood cells fight infections.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
Correct Answer is D
Explanation
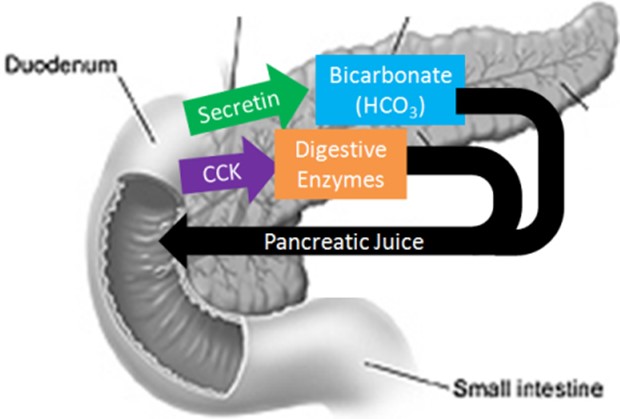
The pancreas secretes large amounts of sodium bicarbonate, which protects the duodenum by neutralizing the acid that comes from the stomach.
This compound helps neutralize stomach acid generated during the digestive process.
Choice A is incorrect because sodium bicarbonate is not a protease that digests carbohydrates.
Proteases are enzymes that break down proteins, while sodium bicarbonate is a chemical compound that helps neutralize stomach acid.
Choice B is incorrect because sodium bicarbonate does not stimulate the pyloric sphincter.
The pyloric sphincter is a ring of smooth muscle that separates the stomach from the duodenum and regulates the passage of partially digested food (chyme) into the small intestine.
Choice C is incorrect because sodium bicarbonate does not inhibit peristalsis.
Peristalsis is a series of wave-like muscle contractions that move food through the digestive tract.
Correct Answer is B
Explanation
Antidiuretic hormone (ADH), also known as vasopressin, is a hormone that helps regulate the amount of water in your body.
It works to control the amount of water your kidneys reabsorb as they filter out waste from your blood.
Choice A is not correct because an increase in the concentration of calcium in the glomerulus is not a physiological response caused by the release of antidiuretic hormone.
Choice C is not correct because a decrease in the concentration of calcium in the glomerulus is not a physiological response caused by the release of antidiuretic hormone.
Choice D is not correct because a decrease in water reabsorption in the collecting duct is not a physiological response caused by the release of antidiuretic hormone.
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
