Before starting antimicrobial therapy, the nurse assesses a patient for possible drug allergy. Which of the following complaints should alert the nurse to a drug allergy?
Headaches
Hives or shortness of breath
Diarrhea
Nausea
The Correct Answer is B
A. Headaches:
Headaches are a common symptom that can occur for various reasons, including stress, tension, dehydration, or as a side effect of medications. While headaches can sometimes occur as a side effect of certain drugs, they are not specific indicators of a drug allergy. Allergic reactions to medications typically involve other symptoms such as rash, hives, itching, swelling, or respiratory symptoms.
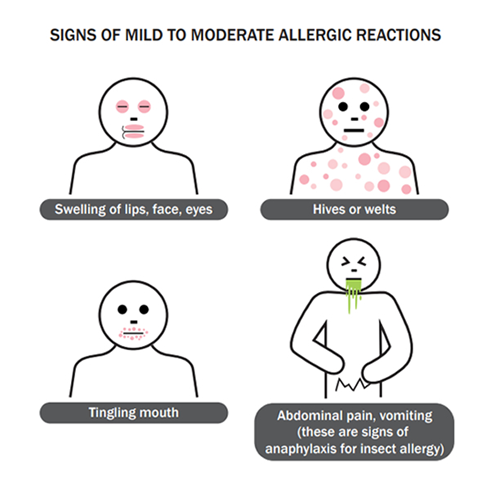
B. Hives or shortness of breath:
Hives (urticaria) are raised, red, itchy welts on the skin that can occur as an allergic reaction to medications. They are a common manifestation of drug allergies. Shortness of breath (dyspnea) can occur as part of a severe allergic reaction known as anaphylaxis. Anaphylaxis is a life-threatening allergic reaction characterized by a rapid onset of symptoms, including difficulty breathing, swelling of the throat or tongue, rapid heart rate, and low blood pressure. Both hives and shortness of breath are significant signs of a potential drug allergy and require immediate attention.
C. Diarrhea:
Diarrhea can occur as a side effect of medications, including antibiotics. However, it is not typically a specific indicator of a drug allergy. Diarrhea is more commonly associated with gastrointestinal disturbances or as a reaction to changes in gut flora due to antibiotic use.
D. Nausea:
Nausea is a common side effect of many medications, including antibiotics. While it can be bothersome, nausea alone is not a specific indicator of a drug allergy. Allergic reactions to medications typically involve other symptoms such as rash, hives, itching, swelling, or respiratory symptoms.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
Correct Answer is B
Explanation
A. 36 months:
This duration is longer than typical treatment courses for TB. While treatment for drug-resistant TB may require an extended duration, standard treatment for drug-sensitive TB typically lasts for a shorter period.
B. 6-12 months:
This duration is within the typical range for the treatment of drug-sensitive TB. Standard treatment regimens for drug-sensitive TB usually involve a combination of multiple antibiotics taken for 6 to 9 months, sometimes extending up to 12 months depending on factors such as the severity of the disease and the patient's response to treatment.
C. 2-4 weeks:
This duration is too short for the treatment of TB. TB treatment requires a prolonged course of antibiotics to ensure the complete eradication of the bacteria and to prevent the development of drug resistance.
D. 7-10 days:
This duration is too short for the treatment of TB. TB treatment typically lasts for several months rather than days, as it involves a combination of antibiotics taken for an extended period to effectively treat the infection.
Correct Answer is D
Explanation
A. Sulfonamides:
Sulfonamides are a class of antibiotics that are structurally distinct from cephalosporins like cefazolin. Allergic reactions to sulfonamides do not necessarily indicate a risk of allergy to cefazolin. However, it's still important to assess for any previous allergic reactions to medications, including sulfonamides, as individuals can have multiple medication allergies.
B. Macrolides:
Macrolides are another class of antibiotics that are structurally different from cephalosporins. Allergic reactions to macrolides do not directly indicate an allergy to cefazolin. However, as with sulfonamides, it's crucial to assess for any history of allergic reactions to medications, including macrolides.
C. Yeast:
Yeast is not a class of antibiotics but rather a type of fungus. Allergic reactions to yeast are unrelated to cephalosporin antibiotics like cefazolin. Therefore, a history of allergic reactions to yeast does not suggest an allergy to cefazolin.
D. Penicillin:
This is the correct choice. Penicillins and cephalosporins share a similar beta-lactam ring structure. Individuals who have had allergic reactions to penicillin may have an increased risk of cross-reactivity with cephalosporins, including cefazolin. Therefore, it's crucial to assess for any previous allergic reactions to penicillin before administering cefazolin to avoid potential allergic reactions or adverse effects.
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
