For an arterial blood gas (ABG) to have full compensation, which of the following statements is correct?
pCO2 and pHCO3 and pH are abnormal but the pa02 remains between 80-100 mmHg
arterial pH & pCO2 are abnormal but the pHCO3 is starting to change
pCO2, pHCO3 and pH have adjusted in expected range in 72 hours
arterial pH is between 7.35-7.45 and the pCO2 & pO2 are abnormal
The Correct Answer is C
C. Full compensation typically occurs within 2 to 3 days (approximately 72 hours) after the onset of an acid-base disturbance. During full compensation, the primary acid-base disorder (e.g., respiratory acidosis or alkalosis, metabolic acidosis or alkalosis) is still present, but the compensatory mechanisms have effectively brought the pH, pCO2, and bicarbonate (pHCO3) levels back towards normal range.
A. Full compensation occurs when both the primary disorder (respiratory or metabolic) and the compensatory mechanism (renal or respiratory) are functioning to return the pH towards normal. In this option, while the pO2 is within the normal range, the pH, pCO2, and bicarbonate (pHCO3) are all abnormal, indicating an ongoing imbalance.
B. Full compensation occurs when all components of the ABG are within or approaching normal range, indicating that the body's compensatory mechanisms have effectively counteracted the primary acid- base disturbance. In this option, the bicarbonate (pHCO3) is mentioned as starting to change, indicating incomplete compensation.
D. While the pH is within the normal range, both the pCO2 and pO2 are abnormal, indicating a primary respiratory disturbance. In the case of full compensation, the pH, pCO2, and bicarbonate (pHCO3) levels would all be within or approaching normal range, indicating that the compensatory mechanisms have effectively counteracted the primary acid-base disturbance.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
Correct Answer is A
Explanation
A. This approach involves providing medication education to the client as each medication is administered. While this ensures that the client receives information about each medication in a timely manner, it may not allow for comprehensive education or adequate time for the client to ask questions or clarify information. Additionally, the client may feel overwhelmed by receiving information about multiple medications at once.
B. Incorporating medication education into another activity, such as assisting the client with his bath, can be an efficient use of time. However, it may not provide an optimal environment for focused learning and discussion. The client may be distracted or uncomfortable during the bath, limiting their ability to absorb and retain information effectively.
C. This approach involves providing medication education to the client after discharge via a follow-up phone call. While this allows for more time and flexibility in providing education, it may not address the client's immediate needs or questions prior to discharge. Additionally, the client may have already started taking the medications by the time of the follow-up call, potentially leading to missed opportunities for clarification or adjustment of the medication regimen.
D. Providing written instructions for the client to read at home is an efficient way to ensure that the client has access to information about their medications. This allows the client to review the information at their own pace and refer back to it as needed. However, written instructions alone may not be sufficient for addressing all aspects of medication education, such as potential side effects, drug interactions, or administration techniques.
Correct Answer is D
Explanation
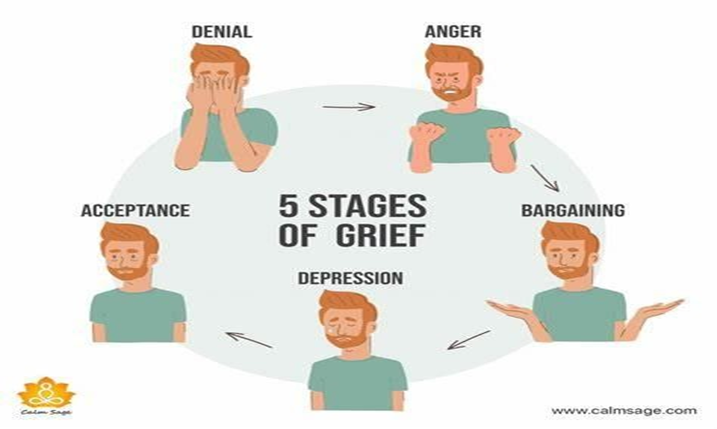
D. Denial is often the initial stage of the grieving process, characterized by disbelief or avoidance of the reality of the situation. Clients may refuse to accept the diagnosis or its implications, clinging to the hope that it is not true. The client's statement of "This cannot be happening to me" is consistent with denial, as they are expressing disbelief or resistance to the reality of their diagnosis.
A. This stage involves feelings of sadness, despair, and hopelessness. While depression is a common response to a terminal diagnosis, the client's statement of "This cannot be happening to me" suggests that they may still be in an earlier stage of grief.
B. Anger is another common stage of the grieving process, characterized by feelings of frustration, resentment, and hostility. Clients may direct their anger towards themselves, others, or even a higher power. While anger can be a prominent reaction to a terminal diagnosis, the client's statement does not explicitly express anger but rather disbelief or resistance.
C. Bargaining is a stage in which individuals may attempt to negotiate or make deals in an effort to change or postpone the inevitable outcome. For example, a client may pray for more time or promise to change their behavior in exchange for a better outcome. The client's statement of "This cannot be happening to me" does not reflect bargaining but rather denial or disbelief.
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
