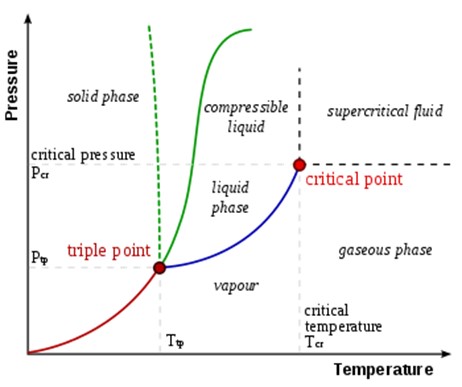
The triple point of a substance is the temperature and pressure at which the substance exists as which of the following?
Simultaneously in sol, gel, and plasma phases
As a gel with solid and liquid trapped in gas
As a sol with gas and solid trapped in liquid
Simultaneously in solid, liquid, and gas phases
The Correct Answer is D
The triple point of a substance is the temperature and pressure at which the substance exists simultaneously in solid, liquid, and gas phases ¹. In thermodynamics, the triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium ¹.
The other options are not correct because they do not accurately describe the triple point of a substance. Sol, gel, and plasma are not phases that coexist at the triple
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
Correct Answer is C
Explanation
. Atoms, cells, tissues, organs. This is the correct order of structures from simple to complex. Atoms are the smallest and simplest units of matter. Cells are made up of atoms and are the basic units of life.
Tissues are groups of similar cells that work together to perform a specific function. Organs are made up of different types of tissues and perform more complex functions.
A. Cells, tissues, atoms, and organs is not in the correct order from simple to complex.
B. Atoms, organs, tissues, cells is not the correct order from simple to complex.
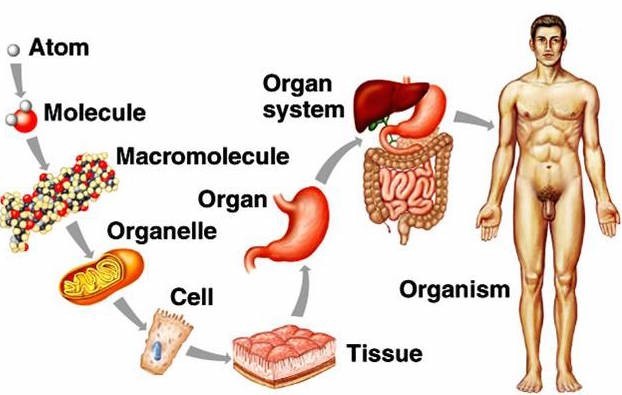
d. Organs, tissues, cells, atoms is not the correct order from simple to complex.
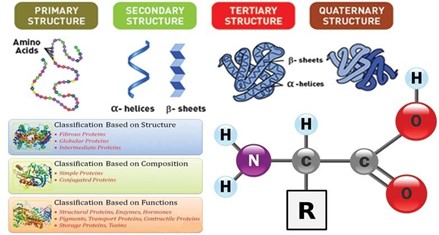
Correct Answer is D
Explanation
Enzymes are a type of protein that catalyze chemical reactions in the body. Proteins are one of the four main classes of biological molecules, along with lipids, carbohydrates, and nucleic acids. The other options are not classes of biological molecules that include enzymes. Lipids are a class of molecules that includes fats and oils, vitamins are organic compounds that are essential for normal growth and nutrition, and carbohydrates are a class of molecules that includes sugars and starches.
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
