Tonicity in cells refers to the concentration of solute in a solution outside of a cell, relative to
The solute concentration of the cytoplasm inside the cell.
The permeability of the cell membrane.
The swelling of the cell.
The number of cell fragments inside the cell.
The presence of a cell wall.
The Correct Answer is A
Choice A rationale: The solute concentration of the cytoplasm inside the cell is correct because tonicity is a measure of how the solution affects the movement of water across the cell membrane by osmosis. Osmosis is the process by which water moves from a region of high water concentration to a region of low water concentration. The water concentration of a solution is determined by the solute concentration of the solution. The higher the solute concentration, the lower the water concentration, and vice versa. Therefore, tonicity compares the solute concentration of the solution outside the cell to the solute concentration of the cytoplasm inside the cell.
Choice B rationale: The permeability of the cell membrane is incorrect because permeability is a property of the cell membrane that determines how easily molecules can pass through it, not a measure of tonicity. The cell membrane is selectively permeable, meaning that it allows some molecules to cross, but not others. The cell membrane is permeable to water, but not to most solutes.
Choice C rationale: The swelling of the cell is incorrect because swelling is a result of tonicity, not a measure of it. Swelling occurs when a cell is placed in a hypotonic solution, which has a higher concentration of water and lower concentration of solute than the cell. In a hypotonic solution, water moves into the cell and out of the solution by osmosis, causing the cell to swell.
Choice D rationale: The number of cell fragments inside the cell is incorrect because cell fragments are pieces of broken cells that have no relation to tonicity. Cell fragments can be produced by mechanical damage, apoptosis, or necrosis, but they do not affect the solute concentration of the cytoplasm or the solution.
Choice E rationale: The presence of a cell wall is incorrect because the cell wall is a structure that surrounds the cell membrane in some cells, such as plant cells, but it does not affect tonicity. The cell wall is made of cellulose, a polysaccharide that is resistant to water. The cell wall provides mechanical support and prevents the cell from bursting in a hypotonic solution, but it does not change the solute concentration of the cytoplasm or the solution.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
Correct Answer is A
Explanation
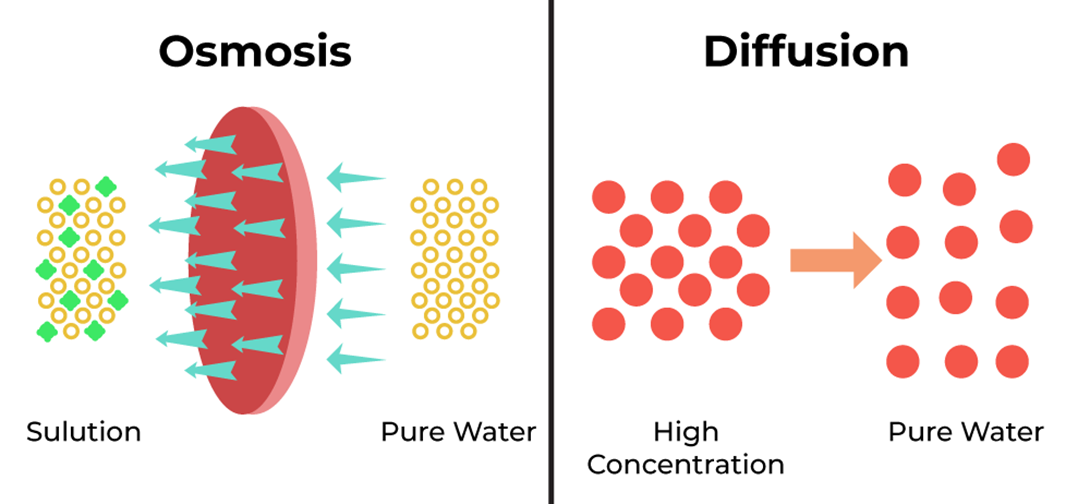
Choice A rationale: Diffusion is correct because it is the passive movement of molecules from an area of high concentration to an area of low concentration. Small lipid soluble molecules can easily cross the plasma membrane by diffusing through the hydrophobic core of the phospholipid bilayer.
Choice B rationale: Filtration is incorrect because it is the process of separating solid particles from a fluid by passing it through a porous medium. Filtration does not involve the plasma membrane, and it does not depend on the solubility of the molecules.
Choice C rationale: Osmosis is incorrect because it is the diffusion of water across a selectively permeable membrane. Osmosis does not apply to lipid soluble molecules, which are not water molecules.
Choice D rationale: Active transport is incorrect because it is the movement of molecules across a membrane against their concentration gradient, which requires energy and transport proteins. Active transport does not depend on the solubility of the molecules, and it is not a passive process.
Choice E rationale: Pumping is incorrect because it is a type of active transport that involves the use of specific pumps to move ions or molecules across a membrane. Pumping does not apply to lipid soluble molecules, which are not ions or polar molecules.
Correct Answer is B
Explanation
Choice A rationale: High power is incorrect because high power is the second highest magnification objective lens, not the highest. High power is also called the 40x objective lens because it magnifies the specimen by 40 times. When combined with the 10x eyepiece lens, the total magnification is 400x.
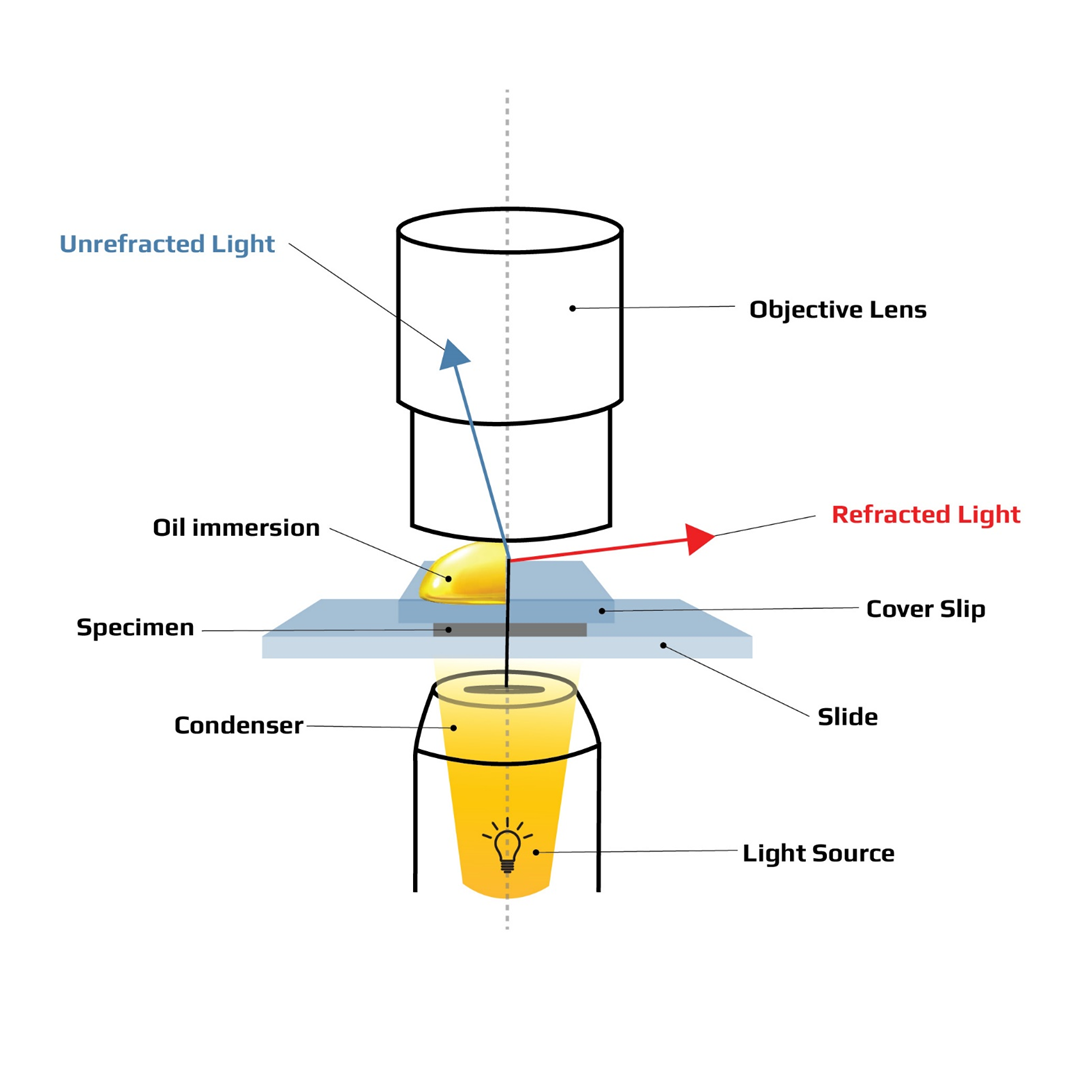
Choice B rationale: Oil immersion is correct because oil immersion is the highest magnification objective lens. Oil immersion is also called the 100x objective lens because it magnifies the specimen by 100 times. When combined with the 10x eyepiece lens, the total magnification is 1000x. Oil immersion requires oil to be applied between the slide and the lens to reduce the refraction of light and increase the clarity of the image.
Choice C rationale: Low power is incorrect because low power is the second lowest magnification objective lens, not the highest. Low power is also called the 10x objective lens because it magnifies the specimen by 10 times. When combined with the 10x eyepiece lens, the total magnification is 100x.
Choice D rationale: Scanning is incorrect because scanning is the lowest magnification objective lens, not the highest. Scanning is also called the 4x objective lens because it magnifies the specimen by 4 times. When combined with the 10x eyepiece lens, the total magnification is 40x. Scanning is used to scan the whole slide and find the specimen.
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
