In a phase diagram, which of the following is the term used for a substance held at a temperature and pressure where the solid, liquid, and gaseous states of a substance exist simultaneously?
Triple point.
Critical temperature.
Critical point.
Absolute zero.
The Correct Answer is A
In a phase diagram, the term used for a substance held at a temperature and pressure where the solid, liquid, and gaseous states of a substance exist simultaneously is the triple point.
The triple point is a unique point on a phase diagram where the three states of matter (solid, liquid, and gas) can coexist in equilibrium.
At the triple point, the temperature and pressure of the substance are fixed.
Option B, critical temperature, is the temperature at which a gas cannot be liquefied, regardless of the pressure applied.
It is a characteristic property of a substance and is typically higher than the boiling point of the liquid at standard pressure.
Option C, critical point, is the point on a phase diagram where the liquid and gas phases of a substance become indistinguishable.
At the critical point, the distinction between the liquid and gas phases disappears, and the substance becomes a supercritical fluid.
Option D, absolute zero, is the theoretical temperature at which all matter has zero thermal energy.
At absolute zero, all substances are in their solid state, but it is not relevant to a phase diagram, as it is a temperature where no transitions between states occur.
In summary, the term used for a substance held at a temperature and pressure where the solid, liquid, and gaseous states of a substance exist simultaneously in a phase diagram is the triple point, whereas the other options provided are not relevant or are characteristic properties of substances in different contexts.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
Correct Answer is D
Explanation
The polarity of water molecules explains its solvent abilities for certain substances.
Water is a polar molecule because it has a partial positive charge on one end and a partial negative charge on the other end due to the unequal sharing of electrons between the oxygen and hydrogen atoms.
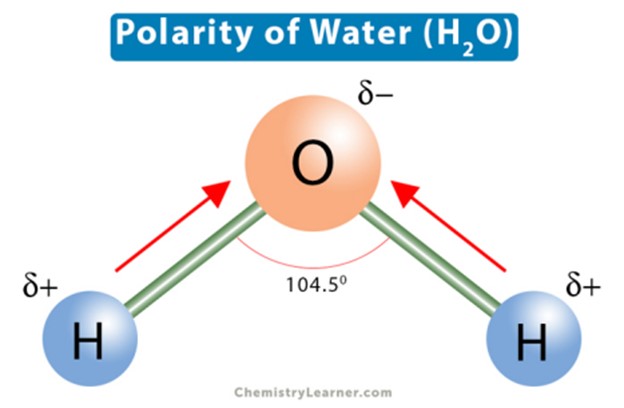
This polarity allows water to dissolve other polar substances and ionic compounds.
Choice A. Kinetic energy of liquid water molecules is not the correct answer because kinetic energy refers to the energy of motion and does not directly explain water’s solvent abilities.
Choice B. High specific heat is not the correct answer because specific heat refers to the amount of heat required to raise the temperature of a substance and does not directly explain water’s solvent abilities.
Choice C. High surface tension is not the correct answer because surface tension refers to the cohesive forces between liquid molecules and does not directly explain water’s solvent abilities.
Correct Answer is B
Explanation
The diameter is the measurement of a straight line passing through the centre of a circle and connecting two points on its circumference.
In this case, the line across the center of the cell represents the diameter of the cell.
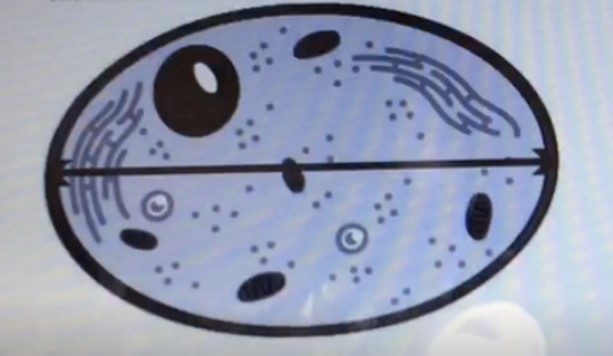
Choice A, Area, is not the correct answer because area refers to the amount of space inside a two-dimensional shape.
Choice C, Volume, is not the correct answer because volume refers to the amount of space occupied by a three-dimensional object.
Choice D, Radius, is not the correct answer because radius refers to the distance from the center of a circle to its circumference and is half the length of the diameter.
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
