The client has an IV of dextrose 5% in 0.45% normal saline. The physician has ordered a transfusion of one unit of packed red blood cells (PRBCs).
Before hanging the blood, the nurse will prime the blood tubing with which of the following solutions?
0.9% sodium chloride.
Lactated Ringer's.
5% dextrose.
5% dextrose in 0.45% sodium chloride.
5% dextrose in 0.45% sodium chloride.
The Correct Answer is A
This is the only solution that is isotonic and compatible with blood products. It will not cause hemolysis or clotting of the blood cells.
Choice B is wrong because lactated Ringer’s is a balanced electrolyte solution that contains calcium, which can cause clotting of the blood cells.
Choice C is wrong because 5% dextrose is a hypotonic solution that can cause hemolysis of the blood cells.
Choice D is wrong because 5% dextrose in 0.45% sodium chloride is a hypertonic solution that can cause hemolysis of the blood cells.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
Correct Answer is ["B","C","D"]
Explanation
Hypercalcemia is a condition in which the calcium level in the blood is above normal.
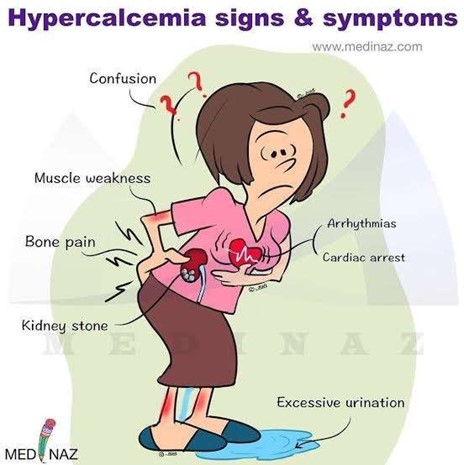
This can cause various symptoms, such as confusion, constipation, and bradycardia (slow heart rate).
These are the clinical manifestations that the nurse would expect to observe in a client with hypercalcemia.
Choice A is wrong because muscle spasms are not a common symptom of hypercalcemia.
In fact, hypercalcemia can cause muscle weakness and pain.
Choice E is wrong because polyuria (excessive urination) is not a direct symptom of hypercalcemia, but rather a result of kidney problems caused by hypercalcemia.
Hypercalcemia can make the kidneys work harder to filter the excess calcium, leading to dehydration and thirst.
However, this does not necessarily mean that the client will have polyuria.
Normal ranges for calcium levels in the blood are 8.5 to 10.2 mg/dL (milligrams per deciliter) or 2.1 to 2.6 mmol/L (millimoles per liter).
Correct Answer is B
Explanation
Hyponatremia is a condition where sodium levels in your blood are lower than normal. This can cause symptoms such as nausea, headache, confusion, muscle weakness and seizures. A hypertonic saline solution is a fluid that has a higher concentration of sodium than normal blood. It can help restore the sodium balance and prevent or treat the complications of hyponatremia.
Choice A is wrong because restricting fluid intake may not be enough to correct severe hyponatremia and may worsen the symptoms if the cause is sodium loss.
Choice C is wrong because encouraging increased fluid intake may further dilute the sodium levels and worsen the condition.
Choice D is wrong because administering a loop diuretic may increase the urine output and cause more sodium loss, leading to more severe hyponatremia.
Normal ranges for blood sodium levels are between 135 and 145 milliequivalents per liter (mEq/L).
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
