Which of the following properties does soap, an emulsifier, have that make it useful for washing dirt off one’s handswith water?
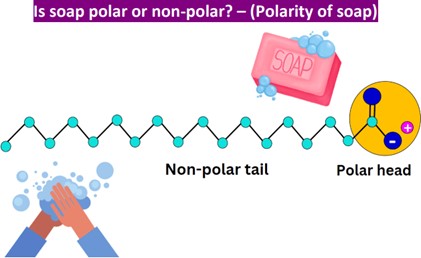
Soap’s dual polar and nonpolar nature helps bond oil and water
Soap’s acidity causes grime to precipitate into the water
Soap’s enzymatic action helps to dissolve grime into small particles
Soap’s rough texture physically scours grime off surfaces
The Correct Answer is A
Soap’s dual polar and nonpolar nature helps bond oil and water. Soap is an emulsifier, which means that it has both polar and nonpolar regions. The polar regions of soap molecules are attracted to water, while the nonpolar regions are attracted to oil and grease. This allows soap to bond with both water and oil, helping to remove dirt and grime from surfaces.
B. Soap’s acidity does not cause grime to precipitate into the water.
C. Soap does not have enzymatic action that helps to dissolve grime into small particles.
D. Soap’s texture does not physically scour grime off surfaces.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
Correct Answer is B
Explanation
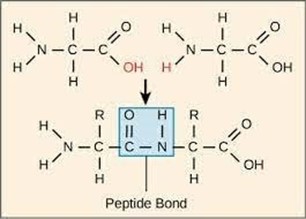
Peptide bonds. Enzymes are proteins, and proteins are made up of amino acid monomers linked together by peptide bonds. A peptide bond is a covalent bond that forms between the carboxyl group of one amino acid and the amino group of another amino acid.
a. Ester bonds are covalent bonds that form between a carboxylic acid and an alcohol.
c. Phosphodiester bonds are covalent bonds that form between a phosphate group and two hydroxyl
groups.
d. Glycosidic bonds are covalent bonds that form between two monosaccharides.
Correct Answer is D
Explanation
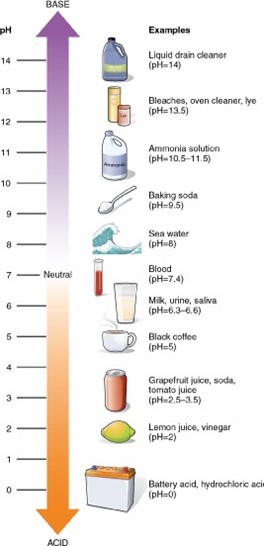
A substance with a pH of 3 is 10 times more acidic than a substance with a pH of 4. The pH scale is a logarithmic scale, which means that each change of one pH unit represents a tenfold change in the hydrogen-ion concentration. A substance with a pH of 3 has a hydrogen-ion concentration that is 10 times greater than that of a substance with a pH of 4.
A. A substance with a pH of 3 is not two times more alkaline than a substance with a pH of 4.
B. A substance with a pH of 3 is not 10 times more alkaline than a substance with a pH of 4.
C. A substance with a pH of 3 is not two times more acidic than a substance with a pH of 4.
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
