Diffusion continues to occur until there is an equal concentration of particles on both sides of a semi-permeable membrane.
True or False?
True
False
The Correct Answer is B
Diffusion is the movement of particles from an area of higher concentration to an area of lower concentration across a semi-permeable membrane. Diffusion stops when there is no net movement of particles across the membrane, which means that the rate of diffusion in one direction is equal to the rate of diffusion in the opposite direction.
This does not necessarily mean that there is an equal concentration of particles on both sides of the membrane, but rather that there is a dynamic equilibrium between the two sides. For example, if a semi-permeable membrane separates a solution of 10% sugar and 90% water from a solution of 20% sugar and 80% water, diffusion will occur until the sugar concentration on both sides is 15%.
However, if the membrane only allows water to pass through, diffusion will occur until the water concentration on both sides is equal, which means that the sugar concentration on one side will be higher than the other. Therefore, diffusion does not always result in an equal concentration of particles on both sides of a semi-permeable membrane.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
Correct Answer is B
Explanation
Choice A rationale: High power is incorrect because high power is the second highest magnification objective lens, not the highest. High power is also called the 40x objective lens because it magnifies the specimen by 40 times. When combined with the 10x eyepiece lens, the total magnification is 400x.
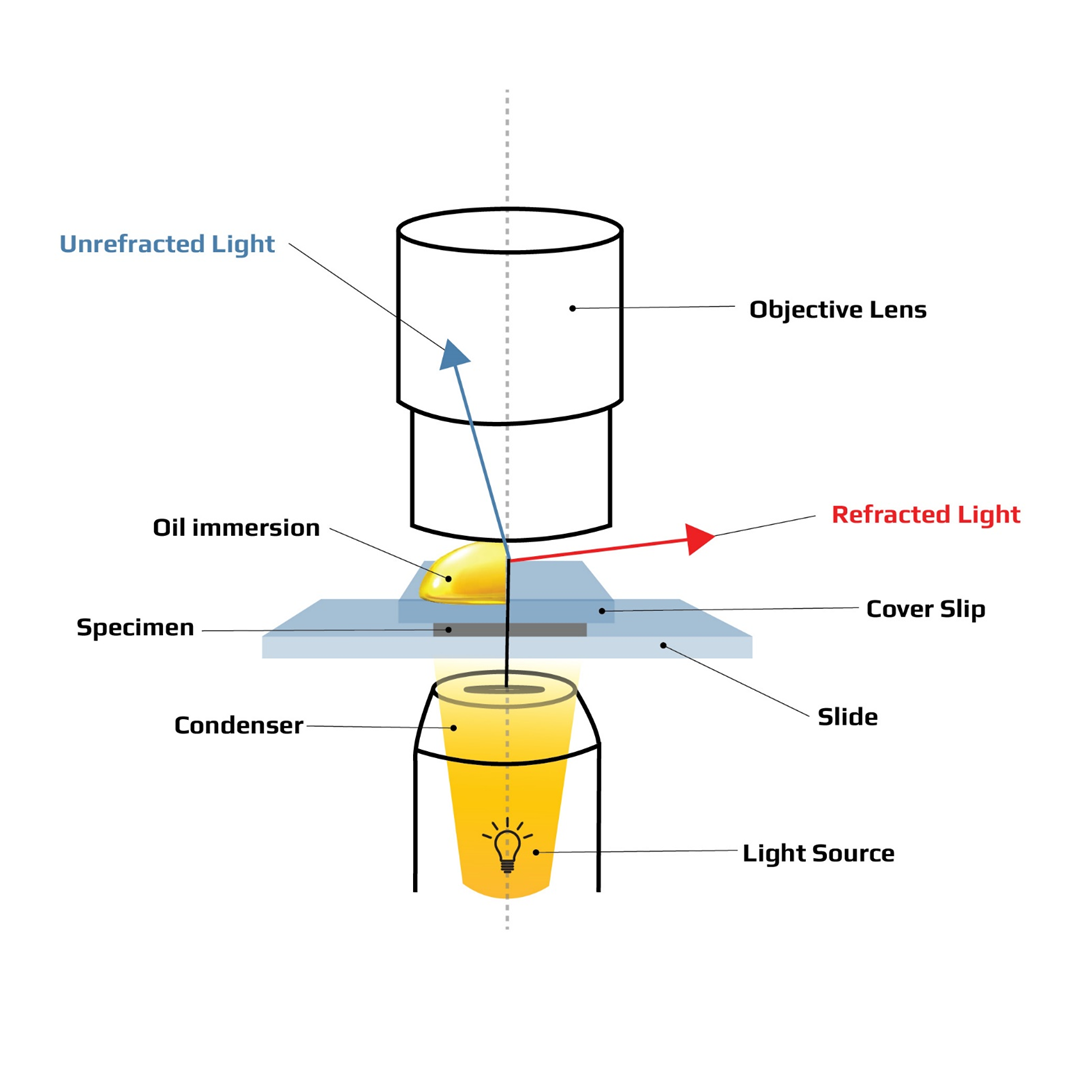
Choice B rationale: Oil immersion is correct because oil immersion is the highest magnification objective lens. Oil immersion is also called the 100x objective lens because it magnifies the specimen by 100 times. When combined with the 10x eyepiece lens, the total magnification is 1000x. Oil immersion requires oil to be applied between the slide and the lens to reduce the refraction of light and increase the clarity of the image.
Choice C rationale: Low power is incorrect because low power is the second lowest magnification objective lens, not the highest. Low power is also called the 10x objective lens because it magnifies the specimen by 10 times. When combined with the 10x eyepiece lens, the total magnification is 100x.
Choice D rationale: Scanning is incorrect because scanning is the lowest magnification objective lens, not the highest. Scanning is also called the 4x objective lens because it magnifies the specimen by 4 times. When combined with the 10x eyepiece lens, the total magnification is 40x. Scanning is used to scan the whole slide and find the specimen.
Correct Answer is D
Explanation
Choice A rationale: CO2 is not the source of oxygen produced by a plant, but a reactant of the dark reaction. The dark reaction uses CO2 and energy intermediates from the light reaction to produce glucose, a type of sugar. The dark reaction does not release any oxygen¹.
Choice B rationale: C6H12O6 is the chemical formula for glucose, which is the product of the dark reaction. Glucose is synthesized from CO2 and energy intermediates from the light reaction. Glucose does not produce any oxygen, but can be used by the plant for respiration or storage².
Choice C rationale: Glyceraldehyde-3-phosphate is an intermediate molecule in the dark reaction. It is formed from CO2 and energy intermediates from the light reaction, and then converted into glucose. Glyceraldehyde-3-phosphate does not produce any oxygen³.
Choice D rationale: H2O is the source of oxygen produced by a plant. In the light reaction, water is split by the energy from sunlight in photosystem II, releasing electrons, protons, and oxygen. The oxygen is either used for respiration or released into the air⁴.
Choice E rationale: O2 is the product of oxygen produced by a plant, not the source. O2 is released as a by-product of the splitting of water in photosystem II. O2 is either used for respiration or released into the air⁴.
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
