Which of the following is correct regarding the pH scale?
A substance with a pH of 3 is two times more alkaline than a substance with a pH of 4.
A substance with a pH of 3 is 10 times more alkaline than a substance with a pH of 4.
A substance with a pH of 3 is two times more acidic than a substance with a pH of 4.
A substance with a pH of 3 is 10 times more acidic than a substance with a pH of 4.
The Correct Answer is D
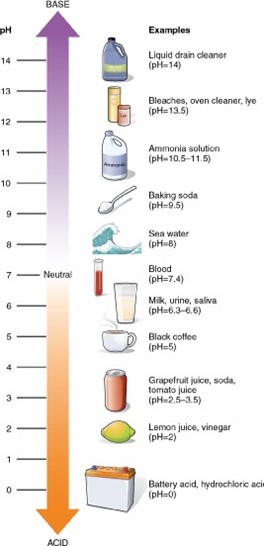
A substance with a pH of 3 is 10 times more acidic than a substance with a pH of 4. The pH scale is a logarithmic scale, which means that each change of one pH unit represents a tenfold change in the hydrogen-ion concentration. A substance with a pH of 3 has a hydrogen-ion concentration that is 10 times greater than that of a substance with a pH of 4.
A. A substance with a pH of 3 is not two times more alkaline than a substance with a pH of 4.
B. A substance with a pH of 3 is not 10 times more alkaline than a substance with a pH of 4.
C. A substance with a pH of 3 is not two times more acidic than a substance with a pH of 4.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
Correct Answer is C
Explanation
. Atoms, cells, tissues, organs. This is the correct order of structures from simple to complex. Atoms are the smallest and simplest units of matter. Cells are made up of atoms and are the basic units of life.
Tissues are groups of similar cells that work together to perform a specific function. Organs are made up of different types of tissues and perform more complex functions.
A. Cells, tissues, atoms, and organs is not in the correct order from simple to complex.
B. Atoms, organs, tissues, cells is not the correct order from simple to complex.
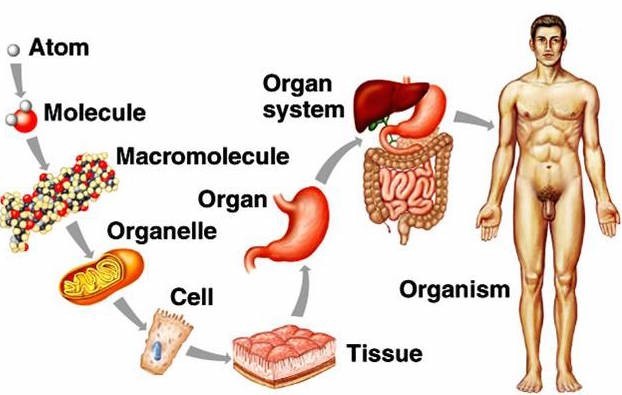
d. Organs, tissues, cells, atoms is not the correct order from simple to complex.
Correct Answer is C
Explanation
The study does not indicate a causal relationship between the processes. A correlation between two processes means that there is a statistical relationship between them, but it does not necessarily imply causation. In other words, just because two processes are correlated does not mean that one causes the other.
A. The study does not indicate that Process A causes Process B.
B. The study can indicate whether Processes A and B have a positive relationship if the correlation is positive.
D. The study can indicate whether Processes A and B have a negative relationship if the correlation is negative.
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
