Which of the following is the effect of inflammation in the healing process?
Pulls the edges of the wound together
Causes bleeding to clean out the wound
Cleans out debris and toxins from the wound
Builds the scab to protect the wound
The Correct Answer is C
a. Pulls the edges of the wound together: Wound closure is a later stage of healing, not a primary function of inflammation.
b. Causes bleeding to clean out the wound: While some bleeding might occur initially, inflammation doesn't actively cause bleeding to clean the wound.
c. Cleans out debris and toxins from the wound: Inflammation is a natural response to injury or infection. It involves increased blood flow, redness, swelling, and heat. This process helps to deliver white blood cells and other immune factors to the area to fight infection and remove debris and damaged tissues, promoting healing.
d. Builds the scab to protect the wound: Scab formation is a result of dried blood and plasma, not a direct effect of inflammation.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
Correct Answer is C
Explanation
a: Basal ganglia - The basal ganglia are involved in motor control and coordination but not in respiratory regulation.
b: Parietal lobe - The parietal lobe is involved in sensory processing and spatial awareness but not in respiratory regulation.
c. Medulla: The medulla oblongata, specifically the respiratory center within it, is responsible for the involuntary control of respiration, including setting the basic rhythm of breathing and responding to changes in blood pH and oxygen levels.
d: Hypothalamus - The hypothalamus regulates various physiological processes, including temperature regulation and hormone secretion, but not respiration.
Correct Answer is C
Explanation
a. Spermatozoa: These are the mature male gametes (sperm cells) that result from spermatogenesis but do not describe the process of meiosis itself.
b. Spermiogenesis: This is the final stage of spermatogenesis, where spermatids undergo morphological changes to become mature spermatozoa.
c. Spermatogenesis: This is the process that includes both meiosis and subsequent maturation steps, resulting in the formation of spermatozoa. It involves the production of haploid sperm cells from diploid germ cells.
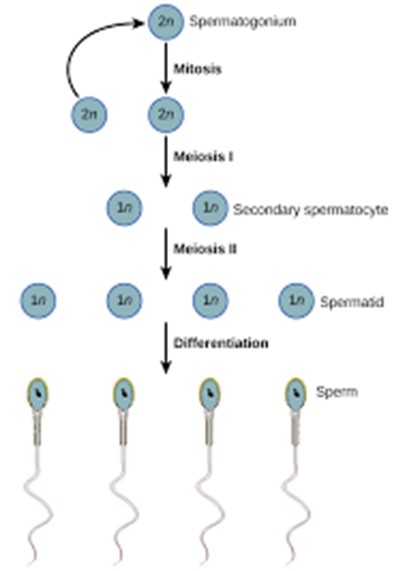
d. Spermatocytes: These are the cells that undergo meiosis during spermatogenesis. Primary spermatocytes undergo the first meiotic division to form secondary spermatocytes, which then undergo the second meiotic division to produce spermatids.
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
